Epigenetic silencing of dual oxidase 1 by promoter hypermethylation in human hepatocellular carcinoma
- PMID: 25232492
- PMCID: PMC4163615
Epigenetic silencing of dual oxidase 1 by promoter hypermethylation in human hepatocellular carcinoma
Abstract
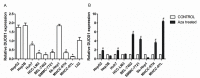
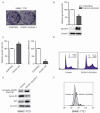
Dual oxidase 1 (DUOX1), which is the main sources for reactive oxygen species (ROS) production in the airway, are frequently silenced in human lung cancer. In poorly differentiated follicular thyroid carcinoma, a high expression of DUOX1 was associated with a reduced risk of death. However, the role of DUOX1 in human hepatocellular carcinoma (HCC) is still not clear. Here, we investigated DUOX1 expression and its promoter methylation status in primary HCC. To date, We found that expression of DUOX1 was decreased significantly in 76.9% (60/78) human hepatocellular carcinoma and 66.7% (6/9) liver cancer cell lines, compared with the paired adjacent non-tumor tissues and immortalized normal cell line. Moreover, which was well correlated with its promoter methylation status. Methylation was further detected in primary HCC, but none or occasionally in paired adjacent non-tumor tissues. Detailed methylation analysis of 35 CpG sites at a 324-bp promoter region by bisulfi te genomic sequencing (BGS) confi rmed its methylation. DUOX1 silencing could be reversed by chemical demethylation treatment with 5-aza-2'-deoxycytidine (5-Aza-dC), indicating direct epigenetic silencing. Restoring DUOX1 expression in lowly expressed cancer cells signifi cantly inhibited cancer cells growth and colony formation ability through the induction of G2/M phase cell cycle arrest and an increase in ROS generation, while knockdown of DUOX1 could markedly promote cancer cells proliferation. In conclusion, we demonstrate that epigenetic silencing of DUOX1 via promoter hypermethylation is common in human liver cancer cells and primary HCC and DUOX1 appears to be a functional tumor suppressor involved in liver carcinogenesis.
Keywords: DUOX1; ROS; hepatocellular carcinoma; methylation.
Figures


References
-
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. - PubMed
-
- Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. - PubMed
-
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. - PubMed
-
- Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. - PubMed
-
- De Deken X, Wang D, Many MC, Costaqliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. - PubMed
LinkOut - more resources
Full Text Sources
