Anticancer properties of novel aminoacetonitrile derivative monepantel (ADD 1566) in pre-clinical models of human ovarian cancer
- PMID: 25232496
- PMCID: PMC4163619
Anticancer properties of novel aminoacetonitrile derivative monepantel (ADD 1566) in pre-clinical models of human ovarian cancer
Abstract
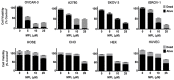
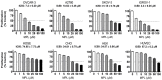
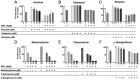
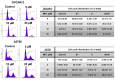
Monepantel (MPL) is a new anthelmintic agent approved for the treatment of nematode infections in farm animals. As a nematicide, it acts through a nematode-specific nicotinic receptor subtype which explains its exceptional safety in rodents and mammals. In the present study, we evaluated its potential as an anticancer agent. In vitro treatment of epithelial ovarian cancer cells with MPL resulted in reduced cell viability, inhibition of cell proliferation and suppression of colony formation. Proliferation of human ovarian surface epithelial cells and other non-malignant cells were however minimally affected. MPL-induced inhibition was found to be independent of the acetylcholine nicotinic receptor (nAChR) indicating that, its target in cancer cells is probably different from that in nematodes. Analysis of MPL treated cells by flow cytometry revealed G1 phase cell cycle arrest. Accordingly, MPL treated cells expressed reduced levels of cyclins D1 and A whereas cyclin E2 expression was enhanced. Consistent with a G1 phase arrest, cellular levels of cyclin dependent kinases (CDKs) 2 and 4 were lower, whereas expression of CDK inhibitor p27(kip) was increased. In cells expressing the wild-type p53, MPL treatment led to increased p53 expression. In line with these results, MPL suppressed cellular thymidine incorporation thus impairing DNA synthesis and inducing cleavage of poly (ADP-ribose) polymerase (PARP-1). Combined these pre-clinical findings reveal for the first time the anticancer potential of monepantel.
Keywords: Monepantel; PARP-1; cell cycle; cyclins; ovarian cancer.
Figures




Similar articles
-
Monepantel induces autophagy in human ovarian cancer cells through disruption of the mTOR/p70S6K signalling pathway.Am J Cancer Res. 2014 Sep 6;4(5):558-71. eCollection 2014. Am J Cancer Res. 2014. PMID: 25232497 Free PMC article.
-
Monepantel antitumor activity is mediated through inhibition of major cell cycle and tumor growth signaling pathways.Am J Cancer Res. 2021 Jun 15;11(6):3098-3110. eCollection 2021. Am J Cancer Res. 2021. PMID: 34249447 Free PMC article.
-
Monepantel is a non-competitive antagonist of nicotinic acetylcholine receptors from Ascaris suum and Oesophagostomum dentatum.Int J Parasitol Drugs Drug Resist. 2018 Apr;8(1):36-42. doi: 10.1016/j.ijpddr.2017.12.001. Epub 2017 Dec 16. Int J Parasitol Drugs Drug Resist. 2018. PMID: 29366967 Free PMC article.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs.Pharmacol Res. 2016 May;107:249-275. doi: 10.1016/j.phrs.2016.03.012. Epub 2016 Mar 16. Pharmacol Res. 2016. PMID: 26995305 Review.
Cited by
-
A preliminary assessment of oral monepantel's tolerability and pharmacokinetics in individuals with treatment-refractory solid tumors.Cancer Chemother Pharmacol. 2020 Nov;86(5):589-594. doi: 10.1007/s00280-020-04146-5. Epub 2020 Sep 22. Cancer Chemother Pharmacol. 2020. PMID: 32960289 Clinical Trial.
-
Synthetic Approaches and Clinical Application of Representative Small-Molecule Inhibitors of Cyclin-Dependent Kinase for Cancer Therapy.Molecules. 2024 Jun 26;29(13):3029. doi: 10.3390/molecules29133029. Molecules. 2024. PMID: 38998978 Free PMC article. Review.
-
Induction of endoplasmic reticulum stress is associated with the anti-tumor activity of monepantel across cancer types.Cancer Med. 2023 Jun;12(12):13522-13537. doi: 10.1002/cam4.6021. Epub 2023 May 6. Cancer Med. 2023. PMID: 37148543 Free PMC article.
-
Physical and chemical factors affecting the loading and release of bromelain from DC beads.Am J Transl Res. 2022 Oct 15;14(10):7135-7146. eCollection 2022. Am J Transl Res. 2022. PMID: 36398211 Free PMC article.
-
Monepantel induces autophagy in human ovarian cancer cells through disruption of the mTOR/p70S6K signalling pathway.Am J Cancer Res. 2014 Sep 6;4(5):558-71. eCollection 2014. Am J Cancer Res. 2014. PMID: 25232497 Free PMC article.
References
-
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, Weber SS, Wenger A, Wieland-Berghausen S, Goebel T, Gauvry N, Pautrat F, Skripsky T, Froelich O, Komoin-Oka C, Westlund B, Sluder A, Maser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. - PubMed
-
- Kaminsky R, Mosimann D, Sager H, Stein P, Hosking B. Determination of the effective dose rate for monepantel (AAD 1566) against adult gastro-intestinal nematodes in sheep. Int J Parasitol. 2009;39:443–446. - PubMed
-
- Rufener L, Baur R, Kaminsky R, Maser P, Sigel E. Monepantel allosterically activates DEG-3/DES-2 channels of the gastrointestinal nematode Haemonchus contortus. Mol Pharmacol. 2010;78:895–902. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
