Thymidylate synthase genotype-directed chemotherapy for patients with gastric and gastroesophageal junction cancers
- PMID: 25232828
- PMCID: PMC4169411
- DOI: 10.1371/journal.pone.0107424
Thymidylate synthase genotype-directed chemotherapy for patients with gastric and gastroesophageal junction cancers
Abstract
Background: Retrospective studies indicate associations between TSER (thymidylate synthase enhancer region) genotypes and clinical outcomes in patients receiving 5-FU based chemotherapy, but well-controlled prospective validation has been lacking.
Methods: In this phase II study (NCT00515216 registered through ClinicalTrials.gov, http://clinicaltrials.gov/show/NCT00515216), patients with "good risk" TSER genotypes (at least one TSER*2 allele) were treated with FOLFOX chemotherapy to determine whether prospective patient selection can improve overall response rates (ORR) in patients with gastric and gastroesophageal junction (GEJ) cancers, compared with historical outcomes in unselected patients (estimated 43%).
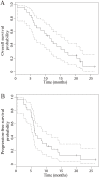
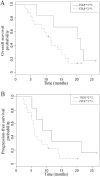
Results: The ORR in genotype-selected patients was 39.1% (9 partial responses out of 23 evaluable patients, 95% CI, 22.2 to 59.2), not achieving the primary objective of improving ORR. An encouraging disease control rate (DCR, consisting of partial responses and stable diseases) of 95.7% was noted and patients with homozygous TSER*2 genotype showed better tumor response.
Conclusions: In this first prospective, multi-institutional study in patients with gastric or GEJ cancers, selecting patients with at least one TSER*2 allele did not improve the ORR but led to an encouraging DCR. Further studies are needed to investigate the utility of selecting patients homozygous for the TSER*2 allele and additional genomic markers in improving clinical outcomes for patients with gastric and GEJ cancers.
Trial registration: ClinicalTrials.gov NCT00515216.
Conflict of interest statement
Figures
References
-
- Brown LM, Devesa SS (2002) Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 11: 235–256. - PubMed
-
- Polednak AP (2003) Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer 105: 98–100. - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, et al. (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697. - PubMed
-
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, et al. (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24: 4991–4997. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

