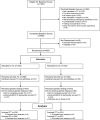
Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling
- PMID: 25232856
- PMCID: PMC4364924
- DOI: 10.1038/gim.2014.125
Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling
Abstract
Purpose: As genetic counseling and testing become more fully integrated into clinical care, alternative delivery models are increasingly prominent. This study examines predictors of genetic testing for hereditary breast/ovarian cancer among high-risk women in a randomized trial of in-person versus telephone-based genetic counseling.
Methods: Methods include multivariable logistic regression and interaction analyses.
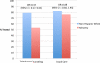
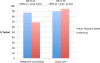
Results: Of the 669 participants, 600 completed counseling and 523 received test results. As previously reported, participants randomized to telephone counseling were significantly less likely to be tested. In intention-to-treat analyses, completion of counseling and testing was associated with: race/ethnicity (odds ratio (OR) = 1.96, 95% confidence interval (CI): 1.20-3.20), perceived stress (OR = 0.89, 95% CI: 0.81-0.98), knowledge (OR = 1.12, 95% CI: 1.02-1.23), and randomization group (OR = 1.48, 95% CI: 1.01-2.16). Further, race/ethnicity moderated the association between randomization group and testing; minority women receiving telephone counseling were least likely to complete testing.
Conclusion: Evidence for logistical and communication-based explanations for this interaction is presented. The overall increased access made possible with telephone genetic counseling should be considered in light of the possibility that this may also lead to lower rates of testing among high-risk minority women. Additional care should be taken to assess and address potential barriers when services are delivered by telephone.Genet Med 17 6, 467-475.
Figures
References
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. V.1.2014. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
-
- U.S. Preventive Services Task Force Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Draft Recommendation Statement. April 2013. AHRQ Publication No. 12-05164-EF-2. http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/draftrecb....
-
- National Society of Genetic Counselors Professional Status Survey: Executive Summary. 2012 http://nsgc.org/p/cm/ld/fid=68.
-
- Brierley KL, Blouch E, Cogswell W, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18(4):303–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous