Transgenic analysis of the Leishmania MAP kinase MPK10 reveals an auto-inhibitory mechanism crucial for stage-regulated activity and parasite viability
- PMID: 25232945
- PMCID: PMC4169501
- DOI: 10.1371/journal.ppat.1004347
Transgenic analysis of the Leishmania MAP kinase MPK10 reveals an auto-inhibitory mechanism crucial for stage-regulated activity and parasite viability
Abstract
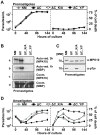
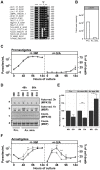
Protozoan pathogens of the genus Leishmania have evolved unique signaling mechanisms that can sense changes in the host environment and trigger adaptive stage differentiation essential for host cell infection. The signaling mechanisms underlying parasite development remain largely elusive even though Leishmania mitogen-activated protein kinases (MAPKs) have been linked previously to environmentally induced differentiation and virulence. Here, we unravel highly unusual regulatory mechanisms for Leishmania MAP kinase 10 (MPK10). Using a transgenic approach, we demonstrate that MPK10 is stage-specifically regulated, as its kinase activity increases during the promastigote to amastigote conversion. However, unlike canonical MAPKs that are activated by dual phosphorylation of the regulatory TxY motif in the activation loop, MPK10 activation is independent from the phosphorylation of the tyrosine residue, which is largely constitutive. Removal of the last 46 amino acids resulted in significantly enhanced MPK10 activity both for the recombinant and transgenic protein, revealing that MPK10 is regulated by an auto-inhibitory mechanism. Over-expression of this hyperactive mutant in transgenic parasites led to a dominant negative effect causing massive cell death during amastigote differentiation, demonstrating the essential nature of MPK10 auto-inhibition for parasite viability. Moreover, phosphoproteomics analyses identified a novel regulatory phospho-serine residue in the C-terminal auto-inhibitory domain at position 395 that could be implicated in kinase regulation. Finally, we uncovered a feedback loop that limits MPK10 activity through dephosphorylation of the tyrosine residue of the TxY motif. Together our data reveal novel aspects of protein kinase regulation in Leishmania, and propose MPK10 as a potential signal sensor of the mammalian host environment, whose intrinsic pre-activated conformation is regulated by auto-inhibition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Zilberstein D, Shapira M (1994) The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 48: 449–470. - PubMed
-
- Doyle PS, Engel JC, Pimenta PF, da Silva PP, Dwyer DM (1991) Leishmania donovani: long-term culture of axenic amastigotes at 37 degrees C. Exp Parasitol 73: 326–334. - PubMed
-
- Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, et al. (2003) An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol 130: 31–42. - PubMed
-
- Kültz D (1998) Phylogenetic and functional classification of mitogen- and stress-activated protein kinases. J Mol Evol 46: 571–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

