HEATR2 plays a conserved role in assembly of the ciliary motile apparatus
- PMID: 25232951
- PMCID: PMC4168999
- DOI: 10.1371/journal.pgen.1004577
HEATR2 plays a conserved role in assembly of the ciliary motile apparatus
Abstract
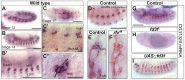
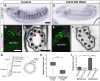
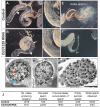
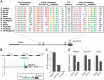
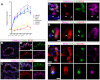
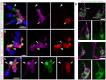
Cilia are highly conserved microtubule-based structures that perform a variety of sensory and motility functions during development and adult homeostasis. In humans, defects specifically affecting motile cilia lead to chronic airway infections, infertility and laterality defects in the genetically heterogeneous disorder Primary Ciliary Dyskinesia (PCD). Using the comparatively simple Drosophila system, in which mechanosensory neurons possess modified motile cilia, we employed a recently elucidated cilia transcriptional RFX-FOX code to identify novel PCD candidate genes. Here, we report characterization of CG31320/HEATR2, which plays a conserved critical role in forming the axonemal dynein arms required for ciliary motility in both flies and humans. Inner and outer arm dyneins are absent from axonemes of CG31320 mutant flies and from PCD individuals with a novel splice-acceptor HEATR2 mutation. Functional conservation of closely arranged RFX-FOX binding sites upstream of HEATR2 orthologues may drive higher cytoplasmic expression of HEATR2 during early motile ciliogenesis. Immunoprecipitation reveals HEATR2 interacts with DNAI2, but not HSP70 or HSP90, distinguishing it from the client/chaperone functions described for other cytoplasmic proteins required for dynein arm assembly such as DNAAF1-4. These data implicate CG31320/HEATR2 in a growing intracellular pre-assembly and transport network that is necessary to deliver functional dynein machinery to the ciliary compartment for integration into the motile axoneme.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Choksi SP, Lauter G, Swoboda P, Roy S (2014) Switching on cilia: transcriptional networks regulating ciliogenesis. Development 141: 1427–1441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_UU_12018/26/MRC_/Medical Research Council/United Kingdom
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- MR/K018558/1/MRC_/Medical Research Council/United Kingdom
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- MR/K018558/10/MRC_/Medical Research Council/United Kingdom
- MR/L01629X/1/MRC_/Medical Research Council/United Kingdom
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102926/HL/NHLBI NIH HHS/United States
- MC_U127561112/MRC_/Medical Research Council/United Kingdom
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- UC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102924/HL/NHLBI NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- UC2 HL102925/HL/NHLBI NIH HHS/United States
- MC_PC_U127561112/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

