The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria
- PMID: 25233271
- PMCID: PMC4169496
- DOI: 10.1371/journal.ppat.1004393
The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria
Abstract
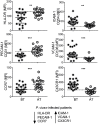
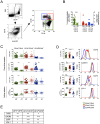
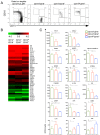
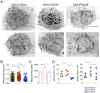
Infection with Plasmodium vivax results in strong activation of monocytes, which are important components of both the systemic inflammatory response and parasite control. The overall goal of this study was to define the role of monocytes during P. vivax malaria. Here, we demonstrate that P. vivax-infected patients display significant increase in circulating monocytes, which were defined as CD14(+)CD16- (classical), CD14(+)CD16(+) (inflammatory), and CD14loCD16(+) (patrolling) cells. While the classical and inflammatory monocytes were found to be the primary source of pro-inflammatory cytokines, the CD16(+) cells, in particular the CD14(+)CD16(+) monocytes, expressed the highest levels of activation markers, which included chemokine receptors and adhesion molecules. Morphologically, CD14(+) were distinguished from CD14lo monocytes by displaying larger and more active mitochondria. CD14(+)CD16(+) monocytes were more efficient in phagocytizing P. vivax-infected reticulocytes, which induced them to produce high levels of intracellular TNF-α and reactive oxygen species. Importantly, antibodies specific for ICAM-1, PECAM-1 or LFA-1 efficiently blocked the phagocytosis of infected reticulocytes by monocytes. Hence, our results provide key information on the mechanism by which CD14(+)CD16(+) cells control parasite burden, supporting the hypothesis that they play a role in resistance to P. vivax infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K (2008) Immunity to malaria: more questions than answers. Nat Immunol 9: 725–732. - PubMed
-
- Spence PJ, Langhorne J (2012) T cell control of malaria pathogenesis. Curr Opin Immunol 24: 444–448. - PubMed
-
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9: 555–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

