Toward a genetic dissection of cortical circuits in the mouse
- PMID: 25233312
- PMCID: PMC4169123
- DOI: 10.1016/j.neuron.2014.08.041
Toward a genetic dissection of cortical circuits in the mouse
Abstract
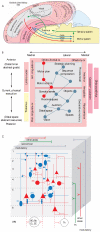
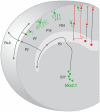
The mammalian neocortex gives rise to a wide range of mental activities and consists of a constellation of interconnected areas that are built from a set of basic circuit templates. Major obstacles to understanding cortical architecture include the diversity of cell types, their highly recurrent local and global connectivity, dynamic circuit operations, and a convoluted developmental assembly process rooted in the genome. With our increasing knowledge of gene expression and developmental genetic principles, it is now feasible to launch a program of genetic dissection of cortical circuits through systematic targeting of cell types and fate mapping of neural progenitors. Strategic design of even a modest number of mouse driver lines will facilitate efforts to compile a cell type parts list, build a Cortical Cell Atlas, establish experimental access to modern tools, integrate studies across levels, and provide coordinates for tracing developmental trajectory from circuit assembly to functional operation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

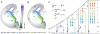



References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Angevine JB, Jr., Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

