An efficient, optimized synthesis of fentanyl and related analogs
- PMID: 25233364
- PMCID: PMC4169472
- DOI: 10.1371/journal.pone.0108250
An efficient, optimized synthesis of fentanyl and related analogs
Abstract
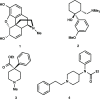
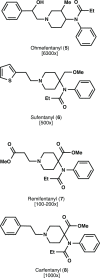
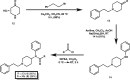
The alternate and optimized syntheses of the parent opioid fentanyl and its analogs are described. The routes presented exhibit high-yielding transformations leading to these powerful analgesics after optimization studies were carried out for each synthetic step. The general three-step strategy produced a panel of four fentanyls in excellent yields (73-78%) along with their more commonly encountered hydrochloride and citric acid salts. The following strategy offers the opportunity for the gram-scale, efficient production of this interesting class of opioid alkaloids.
Conflict of interest statement
Figures
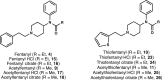

References
-
- Grass JA (1992) Fentanyl: clinical use as postoperative analgesic: epidural/intrathecal route. J. Pain Symptom Manag. 7: 419–430. - PubMed
-
- Grass JA (1992) Sufentanil: clinical use as postoperative analgesic: epidural/intrathecal route. J. Pain Symptom Manag. 7: 271–286. - PubMed
-
- Grass JA (2000) The role of epidural anesthesia and analgesia in postoperative outcome. Anesthesiol. Clin. North America. 18(2): 407–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

