Exploitation of Mycobacterium tuberculosis reporter strains to probe the impact of vaccination at sites of infection
- PMID: 25233380
- PMCID: PMC4169503
- DOI: 10.1371/journal.ppat.1004394
Exploitation of Mycobacterium tuberculosis reporter strains to probe the impact of vaccination at sites of infection
Abstract
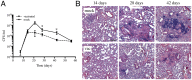
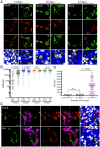
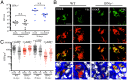
Mycobacterium tuberculosis (Mtb) remains a major public health problem, with an effective vaccine continuing to prove elusive. Progress in vaccination strategies has been hampered by a lack of appreciation of the bacterium's response to dynamic changes in the host immune environment. Here, we utilize reporter Mtb strains that respond to specific host immune stresses such as hypoxia and nitric oxide (hspX'::GFP), and phagosomal maturation (rv2390c'::GFP), to investigate vaccine-induced alterations in the environmental niche during experimental murine infections. While vaccination undoubtedly decreased bacterial burden, we found that it also appeared to accelerate Mtb's adoption of a phenotype better equipped to survive in its host. We subsequently utilized a novel replication reporter strain of Mtb to demonstrate that, in addition to these alterations in host stress response, there is a decreased percentage of actively replicating Mtb in vaccinated hosts. This observation was supported by the differential sensitivity of recovered bacteria to the front-line drug isoniazid. Our study documents the natural history of the impact that vaccination has on Mtb's physiology and replication and highlights the value of reporter Mtb strains for probing heterogeneous Mtb populations in the context of a complex, whole animal model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, et al. (2011) A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17: 189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

