A multinational phase IIb/III trial of beraprost sodium, an orally active prostacyclin analogue, in patients with primary glomerular disease or nephrosclerosis (CASSIOPEIR trial), rationale and study design
- PMID: 25233856
- PMCID: PMC4181382
- DOI: 10.1186/1471-2369-15-153
A multinational phase IIb/III trial of beraprost sodium, an orally active prostacyclin analogue, in patients with primary glomerular disease or nephrosclerosis (CASSIOPEIR trial), rationale and study design
Abstract
Background: Chronic kidney disease (CKD) is public health concern even in Asian countries. TRK-100STP, a sustained release tablet of an orally-active prostacyclin analogue, beraprost sodium, is suggested to suppress worsening of some parameters of renal filtration function, containing in slope of 1/serum creatinine (1/SCr) vs. time in a phase II clinical trial.
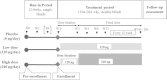
Methods/design: We describe the design of the phase IIb/III trial of TRK-100STP, CASSIOPEIR (CRF Asian Study with Oral PGI2 derivative for Evaluating Improvement of Renal function) conducted in approximately 160 centers in China, Hong Kong, Japan, Malaysia, Republic of Korea, Taiwan, and Thailand. A total of 750 patients (n = 250 per group) with primary glomerular disease or nephrosclerosis were planned to be enrolled. Patients were randomized into one of three treatment groups in a double-bind, placebo-controlled manner: TRK-100STP 60 μg b.i.d.; TRK-100STP 120 μg b.i.d.; or placebo. The treatment period is planned to last 2 to 4 years. The primary efficacy endpoint is the renal composite endpoint including doubling of SCr and ESRD (dialysis induction, renal transplantation, or increase in SCr to ≥ 6.0 mg/dL).
Discussion: This trial targeting CKD patients is designed to (a) demonstrate the superiority of TRK-100STP over placebo using renal composite endpoints, (b) determine the recommended clinical dose, and (c) assess the safety of TRK-100STP in this population and setting.
Trial registration: ClinicalTrials.gov Identifier: NCT01090037.
Figures
References
-
- Akizawa T. Current Status of Dialysis Therapy and Related Clinical Guidelines in Japan. JMAJ. 2010;53(3):185–187.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/15/153/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical