Codeine, alone and with paracetamol (acetaminophen), for cancer pain
- PMID: 25234029
- PMCID: PMC6513650
- DOI: 10.1002/14651858.CD006601.pub4
Codeine, alone and with paracetamol (acetaminophen), for cancer pain
Abstract
Background: Pain is very common in patients with cancer. Opioid analgesics, including codeine, play a significant role in major guidelines on the management of cancer pain, particularly for mild to moderate pain. Codeine is widely available and inexpensive, which may make it a good choice, especially in low-resource settings. Its use is controversial, in part because codeine is not effective in a minority of patients who cannot convert it to its active metabolite (morphine), and also because of concerns about potential abuse, and safety in children.
Objectives: To determine the efficacy and safety of codeine used alone or in combination with paracetamol for relieving cancer pain.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2014, Issue 2), MEDLINE and EMBASE from inception to 5 March 2014, supplemented by searches of clinical trial registries and screening of the reference lists of the identified studies and reviews in the field.
Selection criteria: We sought randomised, double-blind, controlled trials using single or multiple doses of codeine, with or without paracetamol, for the treatment of cancer pain. Trials could have either parallel or cross-over design, with at least 10 participants per treatment group. Studies in children or adults reporting on any type, grade, and stage of cancer were eligible. We accepted any formulation, dosage regimen, and route of administration of codeine, and both placebo and active controls.
Data collection and analysis: Two review authors independently read the titles and abstracts of all studies identified by the searches and excluded those that clearly did not meet the inclusion criteria. For the remaining studies, two authors read the full manuscripts and assessed them for inclusion. We resolved discrepancies between review authors by discussion. Included studies were described qualitatively, since no meta-analysis was possible because of the small amount of data identified, and clinical and methodological between-study heterogeneity.
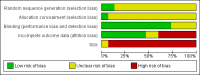
Main results: We included 15 studies including 721 participants with cancer pain due to diverse types of malignancy. All studies were performed on adults; there were no studies on children. The included studies were of adequate methodological quality, but all except for one were judged to be at a high risk of bias because of small study size, and six because of methods used to deal with missing data or high withdrawal rates. Three studies used a parallel group design; the remainder were cross-over trials in which there was an adequate washout period, but only one reported results for treatment periods separately.Twelve studies used codeine as a single agent and three combined it with paracetamol. Ten studies included a placebo arm, and 14 included one or more of 16 different active drug comparators or compared different routes of administration. Most studies investigated the effect of a single dose of medication, while five used treatment periods of one, seven or 21 days. Most studies used codeine at doses of 30 mg to 120 mg.There were insufficient data for any pooled analysis. Only two studies reported our preferred responder outcome of 'participants with at least 50% reduction in pain' and two reported 'participants with no worse than mild pain'. Eleven studies reported treatment group mean measures of pain intensity or pain relief; overall for these outcome measures, codeine or codeine plus paracetamol was numerically superior to placebo and equivalent to the active comparators.Adverse event reporting was poor: only two studies reported the number of participants with any adverse event specified by treatment group and only one reported the number of participants with any serious adverse event. In multiple-dose studies nausea, vomiting and constipation were common, with somnolence and dizziness frequent in the 21-day study. Withdrawal from the studies, where reported, was less than 10% except in two studies. There were three deaths, in all cases due to the underlying cancer.
Authors' conclusions: We identified only a small amount of data in studies that were both randomised and double-blind. Studies were small, of short duration, and most had significant shortcomings in reporting. The available evidence indicates that codeine is more effective against cancer pain than placebo, but with increased risk of nausea, vomiting, and constipation. Uncertainty remains as to the magnitude and time-course of the analgesic effect and the safety and tolerability in longer-term use. There were no data for children.
Conflict of interest statement
Carmen Straube and Kenneth C Jackson have no competing interests to declare. Scott Strassels has received research funding from Endo Pharmaceuticals and Johnson & Johnson in the past two years. Sebastian Straube has received research support from charities, academic and industry sources at various times and has received fees for presentations in the fields of pain and perinatal medicine; none of this was related to this review. Rae Frances Bell has consulted for Pfizer Norway A/S and Grunenthal A/S in a limited capacity, but this was not related to this review. Sheena Derry and Philip J Wiffen have received research support from charities, government, and industry sources at various times but none related to this review.
Figures
Update of
References
References to studies included in this review
Beaver 1978 I {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):92‐100. - PubMed
Beaver 1978 II {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):101‐8. - PubMed
Capretti 1970 {published data only}
-
- Capretti G, Frigerio G. Controlled clinical study of the analgesic activity of a new drug (Z. 424) in pain caused by neoplasms [Studio clinico controllato dell'attivita analgesica di un nuovo farmaco (Z. 424) nel dolore da neoplasie]. La Clinica Terapeutica 1970;52(4):361‐9. - PubMed
Carlson 1990 {published data only}
-
- Carlson RW, Borrison RA, Sher HB, Eisenberg PD, Mowry PA, Wolin EM. A multiinstitutional evaluation of the analgesic efficacy and safety of ketorolac tromethamine, acetaminophen plus codeine, and placebo in cancer pain. Pharmacotherapy 1990;10(3):211‐6. - PubMed
Chen 2003 {published data only}
-
- Chen Y, Zhu W, Liang H, Wu G. The analgesic effect of ibuprofen‐codeine sustained release tablets on postoperative and cancer pain. Chinese Journal of Clinical Rehabilitation 2003;7(8):1290‐1.
Dhaliwal 1995 {published data only}
-
- Dhaliwal HS, Sloan P, Arkinstall WW, Thirlwell MP, Babul N, Harsanyi Z, et al. Randomized evaluation of controlled‐release codeine and placebo in chronic cancer pain. Journal of Pain and Symptom Management 1995;10(8):612‐23. - PubMed
Jochimsen 1978 I {published data only}
-
- Jochimsen PR, Lawton RL, VerSteeg K, Noyes R Jr. Effect of benzopyranoperidine, a delta‐9‐THC congener, on pain. Clinical Pharmacology and Therapeutics 1978;24(2):223‐7. - PubMed
Moertel 1971 {published data only}
-
- Moertel CG, Ahmann DL, Taylor WF, Schwartau N. Aspirin and pancreatic cancer pain. Gastroenterology 1971;60(4):552‐3. - PubMed
Noyes 1975 {published data only}
Rico 2000 {published data only}
-
- Rico MA, Cura MA, Harbst H, Palominos A, Figueroa M, Kramer V. Assessment of tramadol as an alternative opioid instead of codeine at the second step of the WHO analgesic scale [Evaluación de tramadol como un opioide alternativoa la codeína en el segundo peldaño de la escalera analgésica de la OMS]. Revista de la Sociedad Espanola del Dolor 2000;7:345‐53.
Rodriguez 2007 {published data only}
-
- Rodriguez RF, Bravo LE, Castro F, Montoya O, Castillo JM, Castillo MP, et al. Incidence of weak opioids adverse events in the management of cancer pain: a double‐blind comparative trial. Journal of Palliative Medicine 2007;10(1):56‐60. - PubMed
-
- Rodriguez RF, Castillo JM, Castillo MP, Montoya O, Daza P, Rodríguez MF, et al. Hydrocodone/acetaminophen and tramadol chlorhydrate combination tablets for the management of chronic cancer pain: a double‐blind comparative trial. Clinical Journal of Pain 2008;24(1):1‐4. [DOI: 10.1097/AJP.0b013e318156ca4d] - DOI - PubMed
-
- Rodriguez RF, Castillo JM, Pilar Castillo M, Nuñez PD, Rodriguez MF, Restrepo JM, et al. Codeine/acetaminophen and hydrocodone/acetaminophen combination tablets for the management of chronic cancer pain in adults: a 23‐day, prospective, double‐blind, randomized, parallel‐group study. Clinical Therapeutics 2007;29(4):581‐7. - PubMed
Stambaugh 1987 {published data only}
-
- Stambaugh JE Jr, McAdams J. Comparison of the analgesic efficacy and safety oral ciramadol, codeine, and placebo in patients with chronic cancer pain. Journal of Clinical Pharmacology 1987;27(2):162‐6. - PubMed
Staquet 1971 {published data only}
-
- Staquet M, Luyckx A, Cauwenberge H. A double‐blind comparison of alclofenac, pentazocine, and codeine with placebo control in pathologic pain. Journal of Clinical Pharmacology and New Drugs 1971;11(6):450‐5. - PubMed
Staquet 1978 {published data only}
-
- Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical Pharmacology and Therapeutics 1978;23(4):397‐401. - PubMed
Staquet 1993 {published data only}
-
- Staquet M, Renaud A. Double‐blind randomized trial of piroxicam and codeine in cancer pain. Current Theraputics and Research 1993;53(4):435‐40.
References to studies excluded from this review
Chary 1994 {published data only}
-
- Chary S, Goughnour BR, Moulin DE, Thorpe WR, Harsanyi Z, Darke AC. The dose‐response relationship of controlled‐release codeine (Codeine Contin) in chronic cancer pain. Journal of Pain and Symptom Management 1994;9(6):363‐71. - PubMed
Corgill 1965 {published data only}
-
- Corgill DA, Ligon CW, Defelice EA. Double‐blind comparison of namoxyrate, codeine, aspirin, and placebo. Current Therapeutic Research: Clinical and Experimental 1965;7(5):263‐74. - PubMed
De Conno 1991 {published data only}
-
- Conno F, Ripamonti C, Sbanotto A, Barletta L, Zecca E, Martini C, et al. A clinical study on the use of codeine, oxycodone, dextropropoxyphene, buprenorphine, and pentazocine in cancer pain. Journal of Pain and Symptom Management 1991;6(7):423‐7. - PubMed
Jochimsen 1978 II {published data only}
-
- Jochimsen PR, Noyes R Jr. Appraisal of codeine as an analgesic in older patients. Journal of the American Geriatrics Society 1978;26(11):521‐3. - PubMed
Kantor 1966 {published data only}
-
- Kantor TG, Sunshine A, Laska E, Meisner M, Hopper M. Oral analgesic studies: pentazocine hydrochloride, codeine, aspirin, and placebo and their influence on response to placebo. Clinical Pharmacology and Therapeutics 1966;7(4):447‐54. - PubMed
Martinetti 1970 {published data only}
-
- Martinetti L, Lodola E, Monafo V, Ferrari V. Clinical evaluation of an oral analgesic, Z.424, in patients with chronic pain. Journal of Clinical Pharmacology 1970;10(6):390‐9. - PubMed
Minotti 1989 {published data only}
-
- Minotti V, Patoia L, Roila F, Basurto C, Tonato M, Pasqualucci V, et al. Double‐blind evaluation of analgesic efficacy of orally administered diclofenac, nefopam, and acetylsalicylic acid (ASA) plus codeine in chronic cancer pain. Pain 1989;36(2):177‐83. - PubMed
Minotti 1998 {published data only}
-
- Minotti V, Angelis V, Righetti E, Celani MG, Rossetti R, Lupatelli M, et al. Double‐blind evaluation of short‐term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain 1998;74(2‐3):133‐7. - PubMed
Mystakidou 2005 {published data only}
-
- Mystakidou K, Katsouda E, Kouloulias V, Kouvaris J, Tsiatas M, Vlahos L. Comparison of transdermal fentanyl with codeine/paracetamol, in combination with radiotherapy, for the management of metastatic bone pain. Journal of Opioid Management 2005;1(4):2004‐10. - PubMed
Pistevou‐Gompaki 2004 {published data only}
-
- Pistevou‐Gompaki K, Kouloulias VE, Varveris C, Mystakidou K, Georgakopoulos G, Eleftheriadis N, et al. Radiotherapy plus either transdermal fentanyl or paracetamol and codeine for painful bone metastases: a randomised study of pain relief and quality of life. Current Medical Research and Opinion 2004;20(2):159‐63. - PubMed
Sniezek 2011 {published data only}
-
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatologic Surgery 2011;37(7):1007‐13. - PubMed
Stambaugh 1980 {published data only}
-
- Stambaugh JE Jr. Analgesic Equivalence of Tylox® and Percodan®: double‐blind crossover study of patients with pain from malignancy. Current Therapeutic Research 1980;27(2):302‐8.
Sunshine 1988 {published data only}
-
- Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. Journal of Clinical Pharmacology 1988;28(12 Suppl):S47‐54. - PubMed
Watanabe 2008 {published data only}
-
- Watanabe S, Pereira J, Tarumi Y, Hanson J, Bruera E. A randomized double‐blind crossover comparison of continuous and intermittent subcutaneous administration of opioid for cancer pain. Journal of Palliative Medicine 2008;11(4):570‐4. - PubMed
Additional references
Bennett 2003
BNF 2014
-
- Joint Formulary Committee. British National Formulary (online). www.medicinescomplete.com (Accessed on 5 March 2014). London: BMJ Group and Pharmaceutical Press.
Caraceni 2012
-
- Caraceni A, Hanks G, Kaasa S, Bennett M, Brunelli C, Cherny N, et al. for the European Palliative Research Collaborative (EPCRC), on behalf of the European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence‐based recommendations from the EAPC. Lancet Oncology 2012;13(2):e58‐68. [DOI: 10.1016/S1470-2045(12)70040-2] - DOI - PubMed
Cascarbi 2003
-
- Cascarbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. European Journal of Clinical Investigation 2003;33 Suppl 2:17‐22. - PubMed
Ciszkowski 2009
Collins 1997
Cook 1995
De Conno 2003
Derry 2010
Dworkin 2008
Hadley 2013
Higgins 2011
-
- Altman DG, Antes G, Gøtzsche P, Higgins JPT, Jüni P, Lewis S, et al. Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Jackson 2001
-
- Jackson KC, Lipman AG. Opioid analgesics. In: Tollison CD, Satterthwaite JR, Tollison JW editor(s). Practical Pain Management, 3rd Ed. Baltimore, MD: Lippincott, Williams, and Wilkins, 2001.
Jacox 1994
-
- Jacox A, Carr DB, Payne R, Berde CB, Brietbart W, Cain JM, et al. Management of Cancer Pain. Clinical Practice Guideline No. 9. AHCPR Publication No. 94‐0592. Rockville, MD: Agency for Health Care Policy and Research, U.S. Department of Health and Human Services, Public Health Service, 1994.
Jadad 1995
Jadad 1996
Koyyalagunta 2012
-
- Koyyalagunta D, Bruera E, Solanki DR, Nouri KH, Burton AW, Toro MP, et al. A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 2012;15 (3 Suppl):ES39‐58. - PubMed
Kuehn 2013
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Madadi 2007
McNicol 2005
McQuay 1998
-
- McQuay HJ, Moore RA. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998.
Miaskowski 2005
-
- Miaskowski C, Cleary J, Burney R, Coyne P, Finley R, Foster R, et al. Guideline for the Management of Cancer Pain in Adults and Children, APS Clinical Practice Guideline Series, No. 3. Glenview, IL: American Pain Society, 2005.
Moore 1998
Moore 2010
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] - DOI - PubMed
Moore 2013
-
- Moore RA, Derry S, Wiffen PJ. Challenges in design and interpretation of chronic pain trials. British Journal of Anaesthesia 2013;111(1):38‐45. - PubMed
Morris 1995
Nicholson 2007
Nüesch 2010
Opiates 2013
-
- Anon. Codeine. www.opiates.com/codeine/ (Accessed 3 Jan 2014) 2013.
Paul 1989
-
- Paul D, Standifer KM, Inturrisi CE, Pasternak GW. Pharmacological characterization of morphine‐6 beta‐glucuronide, a very potent morphine metabolite. Journal of Pharmacology and Experimental Therapeutics 1989;251(2):477‐83. - PubMed
PCA 2013
-
- Health and Social Care Information Centre, Prescribing, Primary Care. Prescription Cost Analysis, England 2012. The Health and Social Care Information Centre, 2013. [ISBN: 978‐1‐84‐636859‐2]
Quigley 2008
RCPCH 2013
-
- Guidance for the Use of Codeine in Children. Royal College of Anaesthetists, Association of Paediatric Anaesthetists of Great Britain and Ireland, Joint Medicines Committee of the Royal College of Paediatrics and Child Health, Neonatal and Paediatric Pharmacists Group. 2013. [http://www.rcpch.ac.uk/system/files/protected/news/Codeine‐statement‐RCP... (accessed 24 April 2014)]
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ripamonti 2012 I
-
- Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F, ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Annals of Oncology 2012;23 (Suppl 7):vii139‐54. - PubMed
Ripamonti 2012 II
SIGN 2008
-
- Scottish Intercollegiate Guidelines Network. Control of pain in adults with cancer. A national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008.
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Toms 2009
van den Beuken‐van Everdingen 2007
Ventafridda 1985
-
- Ventafridda V, Saita L, Ripamonti C, Conno F. WHO guidelines for the use of analgesics in cancer pain. International Journal of Tissue Reactions 1985;7:93‐6. - PubMed
WHO 1996
-
- World Health Organization. WHO Cancer Pain Relief ‐ a guide to opioid availability, 2nd Edition. Geneva: WHO, 1996.
WHO 2012
-
- World Health Organization. WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. Geneva: WHO, 2012. - PubMed
Wiffen 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical