Aclidinium bromide for stable chronic obstructive pulmonary disease
- PMID: 25234126
- PMCID: PMC8922974
- DOI: 10.1002/14651858.CD010509.pub2
Aclidinium bromide for stable chronic obstructive pulmonary disease
Abstract
Background: Bronchodilators are the mainstay for symptom relief in the management of stable chronic obstructive pulmonary disease (COPD). Aclidinium bromide is a new long-acting muscarinic antagonist (LAMA) that differs from tiotropium by its higher selectivity for M3 muscarinic receptors with a faster onset of action. However, the duration of action of aclidinium is shorter than for tiotropium. It has been approved as maintenance therapy for stable, moderate to severe COPD, but its efficacy and safety in the management of COPD is uncertain compared to other bronchodilators.
Objectives: To assess the efficacy and safety of aclidinium bromide in stable COPD.
Search methods: We identified randomised controlled trials (RCT) from the Cochrane Airways Group Specialised Register of trials (CAGR), as well as www.clinicaltrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), US Food and Drug Administration (FDA) website and Almirall Clinical Trials Registry and Results. We contacted Forest Laboratories for any unpublished trials and checked the reference lists of identified articles for additional information. The last search was performed on 7 April 2014 for CAGR and 11 April 2014 for other sources.
Selection criteria: Parallel-group RCTs of aclidinium bromide compared with placebo, long-acting beta2-agonists (LABA) or LAMA in adults with stable COPD.
Data collection and analysis: Two review authors independently selected studies, assessed the risk of bias, and extracted data. We sought missing data from the trial authors as well as manufacturers of aclidinium. We used odds ratios (OR) for dichotomous data and mean difference (MD) for continuous data, and reported both with their 95% confidence intervals (CI). We used standard methodological procedures expected by The Cochrane Collaboration. We applied the GRADE approach to summarise results and to assess the overall quality of evidence.
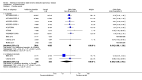
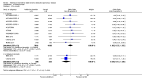
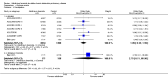
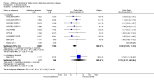
Main results: This review included 12 multicentre RCTs randomly assigning 9547 participants with stable COPD. All the studies were industry-sponsored and had similar inclusion criteria with relatively good methodological quality. All but one study included in the meta-analysis were double-blind and scored low risk of bias. The study duration ranged from four weeks to 52 weeks. Participants were more often males, mainly Caucasians, mean age ranging from 61.7 to 65.6 years, and with a smoking history of 10 or more pack years. They had moderate to severe symptoms at randomisation; the mean post-bronchodilator forced expiratory volume in one second (FEV1) was between 46% and 57.6% of the predicted normal value, and the mean St George's Respiratory Questionnaire score (SGRQ) ranged from 45.1 to 50.4 when reported.There was no difference between aclidinium and placebo in all-cause mortality (low quality) and number of patients with exacerbations requiring a short course of oral steroids or antibiotics, or both (moderate quality). Aclidinium improved quality of life by lowering the SGRQ total score with a mean difference of -2.34 (95% CI -3.18 to -1.51; I(2) = 48%, 7 trials, 4442 participants) when compared to placebo. More patients on aclidinium achieved a clinically meaningful improvement of at least four units decrease in SGRQ total score (OR 1.49; 95% CI 1.31 to 1.70; I(2) = 34%; number needed to treat (NNT) = 10, 95% CI 8 to 15, high quality evidence) over 12 to 52 weeks than on placebo. Aclidinium also resulted in a significantly greater improvement in pre-dose FEV1 than placebo with a mean difference of 0.09 L (95% CI 0.08 to 0.10; I(2) = 39%, 9 trials, 4963 participants). No trials assessed functional capacity. Aclidinium reduced the number of patients with exacerbations requiring hospitalisation by 4 to 20 fewer per 1000 over 4 to 52 weeks (OR 0.64; 95% CI 0.46 to 0.88; I(2) = 0%, 10 trials, 5624 people; NNT = 77, 95% CI 51 to 233, high quality evidence) compared to placebo. There was no difference in non-fatal serious adverse events (moderate quality evidence) between aclidinium and placebo.Compared to tiotropium, aclidinium did not demonstrate significant differences for exacerbations requiring oral steroids or antibiotics, or both, exacerbation-related hospitalisations and non-fatal serious adverse events (very low quality evidence). Inadequate data prevented the comparison of aclidinium to formoterol or other LABAs.
Authors' conclusions: Aclidinium is associated with improved quality of life and reduced hospitalisations due to severe exacerbations in patients with moderate to severe stable COPD compared to placebo. Overall, aclidinium did not significantly reduce mortality, serious adverse events or exacerbations requiring oral steroids or antibiotics, or both.Currently, the available data are insufficient and of very low quality in comparisons of the efficacy of aclidinium versus tiotropium. The efficacy of aclidinium versus LABAs cannot be assessed due to inaccurate data. Thus additional trials are recommended to assess the efficacy and safety of aclidinium compared to other LAMAs or LABAs.
Conflict of interest statement
The authors have no connection with any organisations which could have caused a conflict of interest. We are doing this systematic review for academic purposes.
Figures





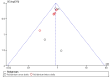
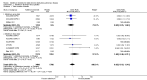









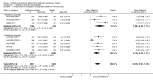


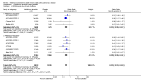



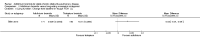
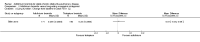
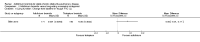
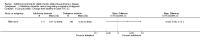





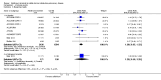
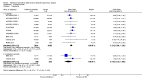
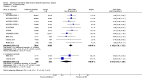


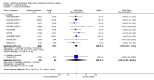

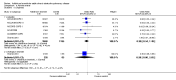
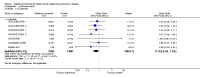

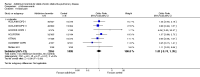

Comment in
-
Aclidinium for Stable COPD.Am Fam Physician. 2015 Jun 1;91(11):760. Am Fam Physician. 2015. PMID: 26034852 No abstract available.
References
References to studies included in this review
ACCLAIM/COPD I {published and unpublished data}
-
- EUCTR2005‐005101‐39‐AT. A 52‐week randomised, double‐blind, parallel group, placebo controlled, multicentre clinical trial, to assess the efficacy and safety of 200 µg of the anticholinergic LAS 34273 compared to placebo, both administered once daily by inhalation, in the maintenance treatment of patients with moderate to severe, stable chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
-
- Jones PW, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic in patients with COPD: ACCLAIM/COPD 1 [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6180 [Poster #207].
-
- Jones PW, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. Efficacy and safety of aclidinium bromide, a novel long‐acting muscarinic antagonist, in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[P2022].
-
- Jones PW, Chanez P, Agusti A, Magnussen H, Fabbri L, Caracta C, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic, in patients with COPD: ACCLAIM/COPD I [Abstract]. Thorax 2009;64 Suppl IV:A168 [P213].
ACCLAIM/COPD II {published and unpublished data}
-
- NCT00358436. Efficacy and safety of LAS 34273 in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT00358436 (accessed 1 May 2014).
-
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. ACCLAIM/COPD II: efficacy and safety of aclidinium bromide; a novel long‐acting muscarinic antagonist in COPD patients, a phase III study [Abstract]. European Respiratory Society Annual Congress Sep 12‐16; Vienna. 2009:[E4351].
-
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. Efficacy and safety of the novel, long‐acting antimuscarinic, aclidinium bromide, in COPD patients in a phase III study: ACCLAIM/COPD II [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6178 [Poster #205].
ACCORD COPD I {published and unpublished data}
-
- D'Urzo A, Make BJ, Kerwin EM, Rekeda L, Sanz MT, Caracta C, et al. Safety and tolerability of twice daily aclidinium bromide in COPD patients: ACCORD COPD I [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1614.
-
- D’Urzo A, Make B, Kerwin E, Rekeda L, Garcia Gil E, Caracta C, et al. ACCORD COPD I: Safety and tolerability of twice daily aclidinium bromide in COPD patients [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38 (55):729s [P3998].
-
- Gelb A, Donohue J, D’Urzo A, Rekeda L, Garcia Gil E, Lateiner J. ACCORD COPD I: Twice‐daily aclidinium bromide improves quality of life and dyspnea in COPD patients [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam 2011;38(55):149s [P876].
-
- Gelb AF, Donohue JF, D'Urzo A, Rekeda L, Jarreta D, Lateiner J. Improvements in quality of life and dyspnea in COPD patients with twice‐daily aclidinium [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1616.
-
- Kerwin E, D'Urzo A, Gelb A, Lakkis H, Garcia Gil E, Caracta C. Twice‐daily aclidinium bromide in COPD patients: Efficacy and safety results from ACCORD COPD I [Abstract]. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1235].
ACCORD COPD II {published and unpublished data}
-
- NCT01045161. To assess the long‐term safety, efficacy and tolerability of inhaled aclidinium bromide in the treatment of moderate‐to‐severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐38). http://clinicaltrials.gov/ct2/show/study/NCT01045161 (accessed 1 May 2014).
-
- Rennard SI, Scanlon PD, Ferguson GT, Rekeda L, Maurer BT, Garcia Gil E, et al. ACCORD COPD II: A randomised clinical trial to evaluate the 12‐week efficacy and safety of twice‐daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clinical Drug Investigation 2013;33(12):893‐904. - PubMed
ACLIFORM {published and unpublished data}
-
- EUCTR2011‐001524‐38‐GB. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combinations compared with individual components and placebo when administered to patients with stable chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
-
- NCT01462942. Long‐term efficacy and safety of aclidinium/formoterol fixed‐dose combination. http://clinicaltrials.gov/show/NCT01462942 (accessed 1 May 2014).
ATTAIN {published and unpublished data}
-
- Agusti A, Jones PW, Bateman E, Singh D, Lamarca R, Miquel G, et al. Improvement in symptoms and rescue medication use with aclidinium bromide in patients with chronic obstructive pulmonary disease: results from ATTAIN [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:149s [P874].
-
- Bateman ED, Singh D, Jones PW, Agusti A, Lamarca R, Miquel G, et al. The ATTAIN study: safety and tolerability of aclidinium bromide in chronic obstructive pulmonary disease [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:730s [P4005].
-
- EUCTR2009‐011600‐27‐CZ. Efficacy and safety of aclidinium bromide at two dose levels vs placebo when administered to patients with moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu....
-
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease (COPD): Reduction in exacerbations as defined by health‐care utilisation and the EXACT diary card [Abstract]. Chest 2011;140(4):529A.
-
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease: Improvement in symptoms and health status in the ATTAIN Study [Abstract]. Chest 2011;140(4):547A.
AUGMENT COPD {published and unpublished data}
-
- D'Urzo A, Mergel V, Leselbaum A, Caracta C. Efficacy and safety of fixed‐dose combination aclidinium bromide/formoterol fumarate in patients with COPD: results from the AUGMENT COPD trial. Chest 2013;144(4):1025A.
-
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The AUGMENT COPD trial: efficacy and safety of a fixed‐dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest 2014;145:426A.
-
- NCT01437397. Efficacy, safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAC). http://clinicaltrials.gov/ct2/show/study/NCT01437397 (accessed 1 May 2014).
Beier 2013 {published and unpublished data}
-
- Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with tiotropium and placebo in patients with moderate‐to‐severe COPD: a phase IIIb study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4253. - PMC - PubMed
-
- Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Improvements in COPD symptoms and rescue medication use with aclidinium bromide compared with tiotropium and placebo: a phase IIIb study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4276.
-
- Beier J, Kirsten AM, Mruz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate‐to‐severe chronic obstructive pulmonary disease: results from a six‐week, randomised, controlled phase IIIb study. COPD 2013;10(4):511‐22. [PUBMED: 23819698] - PMC - PubMed
Chanez 2010 {published and unpublished data}
-
- Chanez P, Burge PS, Dahl R, Creemers J, Chuchalin A, Lamarca R, et al. Aclidinium bromide provides long‐acting bronchodilation in patients with COPD. Pulmonary Pharmacology and Therapeutics 2010;23(1):15‐21. [PUBMED: 19683590] - PubMed
-
- Chanez P, Burge S, Dahl R, Creemers J, Lamarca R, Garcia GE. Once‐daily administration of aclidinium bromide, a novel, long‐acting anticholinergic: a phase II, dose‐finding study [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:[A286].
-
- Charez P, Burge S, Creemers J, Lamarca R, Garcia GE. Once daily administration of aclidinium bromide a novel long acting anticholinergic a phase II dose finding study [Abstract]. European Respiratory Society Annual Congress; Oct 4‐8; Berlin. 2008:[2736].
Maltais 2011 {published and unpublished data}
-
- Casaburi R, Maltais F, Celli B, Porszasz J, Garcia Gil E, Caracta C. Aclidinium bromide improves exercise endurance and decreases exertional dyspnoea in patients with COPD [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22. 2010:[5558].
-
- Celli B, Maltais F, Casaburi R, Porszasz J, Garcia Gil E, Caracta C. Aclidinium bromide improves resting lung function in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22. 2010:[P1183].
-
- Maltais F, Celli B, Casaburi R, Porszasz J, Jarreta D, Seoane B, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Respiratory Medicine 2011;105(4):580‐7. [PUBMED: 21183326] - PubMed
-
- Maltais F, Celli B, Porszasz J, Casaburi R, Garcia GE, Caracta C. Aclidinium bromide improves exercise endurance, dyspnea and inspiratory capacity in patients with moderate to severe COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4428.
-
- NCT00500318. A study of exercise endurance and lung hyperinflation in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT00500318 (accessed 1 May 2014).
NCT01572792 {unpublished data only}
-
- NCT01572792. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28‐weeks treatment in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01572792 (accessed 1 May 2014).
Sliwinski 2010 {published and unpublished data}
-
- EUCTR2007‐004435‐30‐CZ. A randomised, four‐week, placebo‐controlled, double‐blind, six arm parallel group, dose‐finding clinical trial, to assess the efficacy and safety of three different doses of formoterol (6, 12 & 18µg) combined with the inhaled anticholinergic aclidinium bromide 200 µg, aclidinium bromide 200 µg monotherapy and formoterol 12 µg monotherapy all administered once daily by inhalation via Almirall inhaler in patients with stable moderate to severe chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
-
- Sliwinski P, Perng D‐W, Chuchalin A, Jones PW. Efficacy and safety of once‐daily aclidinium bromide 200 µg in combination with formoterol in patients with COPD [Abstract]. Thorax 2010;65 Suppl 4:P137.
References to studies excluded from this review
D'Urzo 2013 {published and unpublished data}
-
- D'Urzo A, Kerwin E, Donohue J, Rennard S, Gelb A, Lakkis H, et al. Effects of twice‐daily aclidinium bromide in COPD patients: A long‐term extension of ACCORD‐COPD I [Abstract]. European Respiratory Society Annual Congress, Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:528s [P2890].
-
- D'Urzo A, Kerwin E, Rennard S, He T, Garcia Gil E, Caracta C. Improvements in lung function with twice‐daily aclidinium bromide: results of a long‐term, phase 3 trial in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2012;142(4_Meeting abstracts):740A.
-
- D'Urzo A, Kerwin E, Rennard S, He T, Garcia Gil E, Caracta C. One‐year extension study of ACCORD COPD I: safety and efficacy of two doses of twice‐daily aclidinium bromide in patients with COPD. COPD 2013;10(4):500‐10. [PUBMED: 23679347] - PubMed
-
- D'Urzo A, Kerwin EM, Donohue JF, Rennard SI, Gelb AF, Lakkis H. Long‐term extension study of ACCORD COPD I: effects of two doses of twice‐daily aclidinium bromide in COPD patients [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2913.
-
- Gelb A, D'Urzo A, Tashkin D, Zhong X, Gil EG, Caracta C. Effects of aclidinium bromide in patients with chronic obstructive pulmonary disease: clinically significant improvements in health status in two one‐year studies. Chest 2012;142(4_Meeting abstracts):691A.
D'Urzo 2013a {unpublished data only}
-
- D'Urzo A, Jones P, Ferguson G, Rekeda L, Gil EG, Caracta C. Aclidinium bromide improves lung function in a wide range of patients with moderate to severe COPD: Pooled subgroup analysis of the ACCORD COPD I and II and ATTAIN trials. Chest 2013;144(4_Meeting abstracts):746A.
D'Urzo 2013b {unpublished data only}
-
- D'Urzo A, Rennard S, Jones P, Rekeda L, Gil EG, Caracta C. Exposure‐adjusted anticholinergic adverse events following long‐term treatment with aclidinium bromide in patients with COPD. Chest 2013;144(4_Meeting abstracts):717A.
de Miquel 2008 {unpublished data only}
-
- Miquel G, Schrodter A, Miletzki B, Gurniak M, Serra C, Jansat JM. Low systematic exposure to aclidinium bromide, a novel long‐acting anticholinergic, after multiple doses [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:Poster #F53.
Donohue 2013 {unpublished data only}
-
- Donohue J, Tashkin D, Ferguson G, Kowey P, Rekeda L, Shrestha P, et al. Long‐term cardiovascular safety of aclidinium bromide in patients with COPD. Chest 2013;144(4_Meeting abstracts):716A.
EUCTR2007‐000010‐36‐DE {unpublished data only}
-
- A multiple dose, double‐blind, double‐dummy, three period cross‐over, placebo controlled clinical trial to assess the efficacy and safety of once daily inhaled aclidinium bromide 200 µg given either in the morning or in the evening in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
EUCTR2007‐003648‐31‐DE {unpublished data only}
-
- A phase IIa, randomised, multicentre, evaluator‐blinded, four‐way crossover clinical trial to study the pharmacokinetics, safety, tolerability and effects on lung function of one day treatment of formoterol 12 µg once daily delivered by two different dry powder inhalers (Aerolizer® and Almirall inhaler), of the fixed dose combination formoterol 12 µg + aclidinium bromide 200 µg once daily delivered by Almirall inhaler, and of formoterol 12 µg twice daily delivered by Aerolizer®, in moderate to severe chronic obstructive pulmonary disease patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
Ferguson 2013 {unpublished data only}
-
- Ferguson G, Kerwin E, Singh D, Kowey P, Rekeda L, Shrestha P, et al. Cardiovascular safety of aclidinium bromide in COPD: pooled results from three placebo‐controlled studies. Chest 2013;144(4_Meeting abstracts):715A.
Flach 2010 {unpublished data only}
-
- Flach S, Jansat JM, Ho J, Garcia GE, Caracta C, Ortiz S. Metabolism and excretion of aclidinium bromide following intravenous administration of [14C] aclidinium bromide in healthy subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4463.
Fuhr 2012 {published and unpublished data}
-
- Fuhr R, Magnussen H, Ribera A, Kirsten A, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P1236].
-
- Fuhr R, Magnussen H, Ribera A, Kirsten A‐M, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide 400 µg compared with placebo and tiotropium 18 µg once daily in moderate to severe COPD patients [Abstract]. Chest 2010;138(4):465A. - PubMed
-
- Fuhr R, Magnussen H, Sarem K, Llovera AR, Kirsten AM, Falques M, et al. Efficacy of aclidinium bromide 400 µg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012;141(3):745‐52. [PUBMED: 21903737] - PubMed
-
- NCT00868231. Efficacy of aclidinium bromide administered in chronic obstructive pulmonary disease (COPD) patients. http://www.clinicaltrials.gov/ct2/show/NCT00868231 (accessed 1 May 2014).
Gelb 2013 {published and unpublished data}
-
- Gelb A, D'Urzo A, Tashkin D, Zhong X, Gil EG, Caracta C. Effects of aclidinium bromide in patients with chronic obstructive pulmonary disease: clinically significant improvements in health status in two 1‐year studies. Chest 2012;142(4_Meeting abstracts):691A.
-
- Gelb A, Tashkin D, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term safety and efficacy of twice‐daily aclidinium bromide in patients with COPD. Respiratory Medicine 2013;107(12):1957‐65. - PubMed
-
- Gelb A, Tashkin D, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term safety of twice‐daily aclidinium bromide in COPD patients: a one‐year, double‐blind study [Abstract]. European Respiratory Society Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40 (Suppl 56):376s [P2118].
-
- Gelb AF, Make BJ, Tashkin DP, Zhong X, Garcia GE, Caracta C. Long‐term efficacy and safety of twice‐daily aclidinium bromide in COPD patients: a one‐year Study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185 (Meeting Abstracts):A2256.
-
- NCT01044459. Long‐term safety, tolerability and efficacy of aclidinium bromide in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐35). http://www.clinicaltrials.gov/ct2/show/study/NCT01044459 (accessed 1 May 2014).
Jansat 2009 {published data only}
-
- Jansat JM, Lamarca R, Garcia Gil E, Ferrer P. Safety and pharmacokinetics of single doses of aclidinium bromide, a novel long‐acting, inhaled antimuscarinic, in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 2009;47(7):460‐8. [PUBMED: 19640353] - PubMed
Jansat 2009a {published data only}
-
- Jansat JM, Lamarca R, Miquel G, Schrodter A, Miletzki B, Gurniak M. Safety and pharmacokinetics of multiple doses of aclidinium bromide, a novel long‐acting muscarinic antagonist for the treatment of chronic obstructive pulmonary disease, in healthy participants. Journal of Clinical Pharmacology 2009;49(10):1239‐46. [PUBMED: 19592595] - PubMed
Joos 2010 {published and unpublished data}
-
- Joos GF, Schelfhout VJ, Pauwels RA, Kanniess F, Magnussen H, Lamarca R, et al. Bronchodilatory effects of aclidinium bromide, a long‐acting muscarinic antagonist, in COPD patients. Respiratory Medicine 2010;104(6):865‐72. [PUBMED: 20044242] - PubMed
-
- Joss G, Schelflout V, Kanniess F, Ludwig‐Segpiel A, Garcia Gil E, Massana Montejo E. Bronchodilator effects of aclidinium bromide a novel long‐acting anticholinergic in COPD patients a phase II study [Abstract]. European Respiratory Journal 2007;30 Suppl 51:210s [1299].
Kerwin 2013 {unpublished data only}
-
- EUCTR2009‐015901‐38‐DE. Efficacy, safety and tolerability of two fixed‐dose combinations of aclidinium bromide with two doses of formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo all administered twice daily in stable, moderate to severe chronic obstructive pulmonary disease patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
-
- Kerwin E, Lapidus R, Leselbaum A, Ortiz S, Rowe P, Caracta C. Dose‐ranging study of two fixed‐dose combinations of twice‐daily aclidinium bromide plus formoterol in patients with moderate to severe COPD. Chest 2013;144(4_Meeting Abstracts):747A.
-
- NCT01049360. Efficacy and safety study of two fixed‐dose combinations of aclidinium bromide with formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo (LAC‐MD‐27). http://clinicaltrials.gov/ct2/show/NCT01049360 (accessed 1 May 2014).
Lasseter 2008 {unpublished data only}
-
- Lasseter KC, Aubets J, Chuecos F, Garcia GE. Aclidinium bromide, a long‐acting antimuscarinic, does not affect QT interval in healthy subjects. Journal of Clinical Pharmacology 2011;51(6):923‐32. - PubMed
-
- Lasseter KC, Aubets J, Garcia GE. Aclidinium bromide a novel long acting anticholinergic does not affect QT interval in healthy subjects [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A655[#F54].
Lasseter 2012 {published and unpublished data}
-
- Lasseter K, Dilzer S, Jansat JM, Garcia GE, Caracta CF, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers. Pulmonary Pharmacology and Therapeutics 2012;25(2):193‐9. [PUBMED: 22366196] - PubMed
-
- Lasseter K, Dilzer S, Jansat JM, Garcia Gil E, Caracta C, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1615. - PubMed
Magnussen 2009 {published data only}
-
- Magnussen H, Watz H, Zimmermann I, Macht S, Greguletz R, Falques M, et al. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respiratory Medicine 2009;103(12):1832‐7. [PUBMED: 19651504] - PubMed
Magnussen 2010 {unpublished data only}
-
- EUCTR2008‐006886‐10‐DE. A multiple dose, double blind, double‐dummy, two‐week three way cross‐over, placebo‐controlled clinical trial to assess the efficacy and safety of twice daily inhaled aclidinium bromide 400 µg compared to placebo and to an active comparator in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 1 May 2014).
-
- Magnussen H, Ribera LA, Kirsten AM, Falques M, Caracta C, Garcia GE. Efficacy and safety of aclidinium bromide 400 {micro}g twice daily compared with placebo and tiotropium in patients with moderate to severe COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4440.
NCT00435760 {unpublished data only}
-
- EUCTR2005‐005804‐17‐NL. A single dose, double‐blind, double‐dummy, three period cross‐over, placebo controlled clinical trial to assess the rate of onset of action of inhaled LAS 34273 200 µg compared to placebo and tiotropium 18 µg in patients with chronic obstructive pulmonary disease (COPD). http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2005‐005804‐17‐NL or https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu....
-
- NCT00435760. Clinical trial to assess rate of onset of bronchodilator action in severe stable chronic obstructive pulmonary disease (COPD) patients. http://clinicaltrials.gov/ct2/show/study/NCT00435760 (accessed 2 May 2014).
NCT00626522 {unpublished data only}
-
- NCT00626522. Aclidinium/formoterol fixed combination dose finding study. http://clinicaltrials.gov/ct2/show/NCT00626522 (accessed 2 May 2014).
NCT00706914 {unpublished data only}
-
- NCT00706914. Comparison of aclidinium bromide and formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT00706914 (accessed 2 May 2014).
NCT01078623 {unpublished data only}
-
- NCT01078623. Efficacy and safety of two fixed dose combinations of aclidinium bromide with formoterol fumarate (ALIGHT‐COPD). http://clinicaltrials.gov/show/NCT01078623 (accessed 2 May 2014).
NCT01437540 {published and unpublished data}
-
- Make B, Donohue J, Zhong X, Leselbaum A, Caracta C. Long‐term safety of a fixed‐dose combination of aclidinium bromide/formoterol fumarate in patients with stable moderate to severe COPD. Chest 2014;145:386A.
-
- NCT01437540. Safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (LAC). http://clinicaltrials.gov/ct2/show/study/NCT01437540 (accessed 2 May 2014).
NCT01551888 {unpublished data only}
-
- NCT01551888. Pharmacokinetic, safety and tolerability study of aclidinium/formoterol fixed dose combination and formoterol in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01551888 (accessed 2 May 2014).
NCT01908140 {unpublished data only}
-
- EUCTR2013‐000116‐14‐HU. A randomised, double‐blind, double‐dummy, active‐controlled study evaluating the efficacy, safety and tolerability of twice‐daily aclidinium bromide /formoterol fumarate compared with twice‐daily salmeterol/fluticasone propionate for 24‐weeks treatment in symptomatic patients with chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 2 May 2014).
-
- NCT01908140. Study of aclidinium bromide/formoterol fumarate compared with salmeterol/fluticasone propionate in patients with chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01908140 (accessed 2 May 2014).
NCT01915784 {unpublished data only}
-
- NCT01915784. Preference, satisfaction and ease of use of Genuair® (Pressair™) and Breezhaler® (Neohaler™) inhalers in patients with COPD. http://clinicaltrials.gov/ct2/show/NCT01915784 (accessed 2 May 2014).
NCT02038829 {unpublished data only}
-
- NCT02038829. A dose‐range finding study of SUN‐101 in subjects with moderate to severe COPD (GOLDEN 6). http://clinicaltrials.gov/ct2/show/NCT02038829 (accessed 2 May 2014).
NCT02039050 {unpublished data only}
-
- NCT02039050. Evaluation of long‐acting muscarinic antagonists in COPD (MAN04). http://clinicaltrials.gov/ct2/show/NCT02039050 (accessed 2 May 2014).
Ortiz 2010 {unpublished data only}
-
- Ortiz S, Flach S, Caracta C, Garcia GE, Jansat JM. Safety and tolerability of aclidinium bromide administered intravenously and absolute bioavailability of inhaled aclidinium bromide in healthy subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4464.
-
- Ortiz S, Flach S, Caracta C, Garcia Gil E, Jansat J. Absolute bioavailability of inhaled aclidinium bromide and safety and tolerability of aclidinium bromide administered intravenously in healthy subjects [Abstract]. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1180].
Schelfhout 2010 {published and unpublished data}
-
- Schelfhout VJ, Joos GF, Ferrer P, Kannies F, Luria X, Richter K, et al. Activity of LAS34273, a new long acting anticholinergic antagonist, in COPD patients [abstract]. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003; Vol. B024 Poster 44.
-
- Schelfhout VJ, Joos GF, Garcia Gil E, Montejo EM. Bronchodilator/broncho‐protective effects of aclidinium bromide, a novel long‐acting anticholinergic:a phase I study. European Respiratory Journal 2007;30 Suppl 51:356S.
Singh 2012 {published and unpublished data}
-
- Efficacy and safety of three doses of aclidinium bromide compared to placebo and to an active comparator in chronic obstructive pulmonary disease (COPD) patients. http://www.clinicaltrials.gov/ct2/show/NCT01120093 (accessed 2 May 2014).
-
- EUCTR2009‐017380‐42‐DE. Efficacy and safety of three doses of aclidinium bromide compared to placebo and to an active comparator all administered twice daily by inhalation in patients with stable moderate and severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 2 May 2014).
-
- Singh D, Magnussen H, Kirsten A, Mindt S, Caracta C, Seoane B, et al. A randomised, placebo‐ and active‐controlled dose‐finding study of aclidinium bromide administered twice a day in COPD patients. Pulmonary Pharmacology and Therapeutics 2012;25(3):248‐53. [PUBMED: 22497752] - PubMed
-
- Singh D, Magnussen H, Kirsten A, Mindt S, Caracta C, Seoane B, et al. Corrigendum to " A randomised, placebo‐ and active‐controlled dose‐finding study of aclidinium bromide administered twice a day in COPD patients" [Pulm Pharmacol Ther 25 (3) (2012) 248‐53]. Pulmonary Pharmacology and Therapeutics 2013;26(2):305. - PubMed
-
- Singh D, Magnussen H, Kirsten A, Mindt‐Pruefert S, Caracta C, Jarreta D, et al. Aclidinium bromide: a phase IIB, dose‐finding study [Abstract]. Thorax 2011;66 Suppl 4:A172 [P256].
van der Palen 2013 {published and unpublished data}
-
- NCT01385696. Study evaluating preference, satisfaction and ease of use of inhalers in chronic obstructive pulmonary disease (COPD) diagnosed patients. http://clinicaltrials.gov/ct2/show/NCT01385696 (accessed 2 May 2014).
-
- Palen J, Ginko T, Kroker A, Valk P, Goosens M, Padulles L, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opinion on Drug Delivery 2013;10(8):1023‐31. - PubMed
-
- Palen J, Ginko T, Kroker A, Valk P, GoosensM, Padulles L, et al. Preference, satisfaction and critical errors with Genuair® and HandiHaler® in patients with COPD. European Respiratory Journal 2012;40 Suppl 56:389s.
Vestbo 2010 {published and unpublished data}
-
- Vestbo J, Vogelmeier C, Creemers J, Falques M, Ribera A, Gil EG. Onset of effect of aclidinium, a novel, long‐acting muscarinic antagonist, in patients with COPD. COPD 2010;7(5):331‐6. [PUBMED: 20854047] - PubMed
-
- Vestbo J, Vogelmeier C, Creemers J, Ribera A, Garcia Gil E. Fast onset of effect of aclidinium bromide, a novel long‐acting muscarinic antagonist, in patients with COPD [Abstract]. Thorax 2009;64 Suppl IV:A167 [P212]. - PubMed
-
- Vestbo J, Vogelmeier C, Creemers J, Ribera A, Garcia Gil E. Rate of onset of action of aclidinium bromide, a novel, long‐acting, muscarinic antagonist [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E4362].
Watz 2013 {unpublished data only}
-
- Beeh KM, Watz H, Magnussen H, Puente‐Maestu L, Jarreta D, Caracta C, et al. Aclidinium bromide improves exercise endurance and dynamic hyperinflation and decreases exertional dyspnoea in patients with moderate‐to‐severe COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2430.
-
- Beeh KM, Watz H, Magnussen H, Puente‐Maestu L, Jarreta D, Caracta C, et al. Effects of aclidinium bromide on exercise endurance, dynamic hyperinflation, physical activity and exertional dyspnoea in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress, Sep 7‐11; Barcelona. 2013; Vol. 42 Suppl 57:636s [3035].
-
- EUCTR2011‐002665‐38‐DE. A multiple dose, randomised, double‐blind, placebo controlled, two period crossover clinical trial to assess the effect of aclidinium bromide 400 µg bid on exercise endurance in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu... (accessed 2 May 2014).
-
- NCT01471171. Efficacy and safety of aclidinium bromide 400 µg BID (twice a day) compared to placebo in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/show/NCT01471171 (accessed 2 May 2014).
-
- Watz H, Beeh KM, Magnussen H, Teres L, Jarreta D, Caracta C, et al. Aclidinium bromide improves static lung function and hyperinflation in patients with moderate‐to‐severe COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2431.
References to studies awaiting assessment
NCT01636401 {unpublished data only}
-
- NCT01636401. Efficacy and safety of 400 μg twice daily of aclidinium bromide vs. placebo when administered to patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01636401 (accessed 1 May 2014).
References to ongoing studies
ASCENT COPD {unpublished data only}
-
- NCT01966107. Evaluate the effect of aclidinium bromide on long‐term cardiovascular safety and COPD exacerbations in patients with moderate to very severe COPD (ASCENT COPD). http://clinicaltrials.gov/ct2/show/NCT01966107 (accessed 1 May 2014).
Additional references
Alagha 2011
-
- Alagha K, Bourdin A, Tummino C, Chanez P. An update on the efficacy and safety of aclidinium bromide in patients with COPD. Therapeutic Advances in Respiratory Disease 2011;5(1):19‐28. [PUBMED: 20884687] - PubMed
Alagha 2014
Almirall
-
- Almirall. Clinical trial results. http://www.almirall.com/webcorp2/cda/ImD_04_03.jsp?fMarc= (accessed 11 April 2014).
ATS/ERS 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. - PubMed
CDC 2011
-
- Hoyert DL, Xu J. Deaths: preliminary data for 2011 (National Vital Statistics Reports). http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf (accessed 25 December 2012). - PubMed
Chong 2011
Chong 2012
Cranston 2008
-
- Cranston JM, Crockett A, Moss J, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001744.pub2] - DOI
Effing 2009
FDA 2012
-
- United States Food, Drug Administration (FDA). Drug approval package; Tudorza Pressair (aclidinium bromide) inhalation powder. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202450Orig1s000TO... (accessed 25 December 2012).
Forest Pharmaceuticals
-
- Forest Pharmaceuticals, Inc. Subsidiary of Forest Laboratories, Inc. http://www.forestpharm.com/ (accessed 25 December 2012).
Gavaldà 2010
-
- Gavaldà A, Garcia‐Gil E. Aclidinium bromide, a novel long‐acting muscarinic antagonist (LAMA). New drugs and targets for asthma and COPD. Progress in Respiratory Research 2010;39:33‐8.
GOLD 2013
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. Global strategy for diagnosis, management and prevention of COPD. http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐managem... (accessed 14 February 2013).
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. www.cochrane‐handbook.org.
Hogg 2009
-
- Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annual Review of Pathology: Mechanisms of Disease 2009;4:435‐59. - PubMed
Holland 2012
Jones 2011
-
- Jones PW, Rennard SI, Agusti A, Chanez P, Magnussen H, Fabbri L, et al. Efficacy and safety of once‐daily aclidinium in chronic obstructive pulmonary disease. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3098801/ (accessed 25 December 2012). [DOI: 10.1186/1465-9921-12-55] - DOI - PMC - PubMed
Jones 2012
-
- Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, Miquel G, et al. Efficacy and safety of twice‐daily aclidinium bromide in COPD patients: the ATTAIN study. European Respiratory Journal 2012;40(4):830‐6. [PUBMED: 22441743] - PubMed
Jones 2013
-
- Jones P. Aclidinium bromide twice daily for the treatment of chronic obstructive pulmonary disease: a review. Advances in Therapy 2013;30(4):354‐68. [PUBMED: 23553509] - PubMed
Karabis 2013
-
- Karabis A, Lindner L, Mocarski M, Huisman E, Greening A. Comparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta‐analysis. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:405‐23. [PUBMED: 24043936] - PMC - PubMed
Karakiulakis 2012
-
- Karakiulakis G, Roth M. Muscarinic receptors and their antagonists in COPD: anti‐inflammatory and anti‐remodeling effects. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512336/ (accessed 25 December 2012). [DOI: 10.1155/2012/409580] - DOI - PMC - PubMed
Karner 2012
Lacasse 2009
MacNee 2006
-
- MacNee W. ABC of chronic obstructive pulmonary disease: pathology, pathogenesis, and pathophysiology. BMJ 2006;332:1202‐4.
Maltais 2012
-
- Maltais F, Milot J. The potential for aclidinium bromide, a new anticholinergic, in the management of chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2012;6(6):345‐61. [PUBMED: 23075544] - PubMed
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] - DOI
NICE 2010
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. http://guidance.nice.org.uk/CG101/Guidance/pdf/English (accessed 25 December 2012).
NICE 2011
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. http://guidance.nice.org.uk/CG101/CostingReport/pdf/English (accessed 25 December 2012).
Poole 2010
-
- Poole P, Chacko EE, Wood‐Baker R, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD002733.pub2] - DOI
Poole 2012
Ram 2009
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sims 2011
Suppli 2012
Sutherland 2004
-
- Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 2004;350(26):2689‐97. [PUBMED: 15215485] - PubMed
Tiong 2009
TSANZ 2012
-
- Abramson M, Crockett AJ, Dabscheck E, Frith PA, George J, Glasgow N, et al. on behalf of Lung Foundation Australia and the Thoracic Society of Australia and New Zealand. The COPD‐X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. Version 2.35 2013. http://www.copdx.org.au/ (accessed 14 February 2014).
van der Meer 2012
Vogelmeier 2011
-
- Vogelmeier C, Banerji D. NVA237, a long‐acting muscarinic antagonist, as an emerging therapy for chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2011;5(3):163‐73. [PUBMED: 21511677] - PubMed
Walters 2009
Walters 2010
Welsh 2011
-
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007891.pub2] - DOI
WHO 2008
-
- World Health Organization. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004updat... (accessed 25 December 2012).
WHO 2010
-
- World Health Organization. Global status report on noncommunicable diseases 2010. http://www.who.int/nmh/publications/ncd_report2010/en/ (accessed 25 December 2012).
WHO 2012
-
- World Health Organization. Chronic obstructive pulmonary disease. http://www.who.int/respiratory/copd/en (accessed 25 December 2012).
WHO 2012a
-
- World Health Organization. COPD predicted to be third leading cause of death in 2030. http://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/inde... (accessed 25 December 2012).
WHO ICTRP
-
- World Health Organization. International Clinical Trials Registry Platform (ICTRP) search portal. http://apps.who.int/trialsearch/ (accessed 2 May 2014).
Woods 2013
-
- Woods JA, Nealy KL, Barrons RW. Aclidinium bromide: an alternative long‐acting inhaled anticholinergic in the management of chronic obstructive pulmonary disease. The Annals of Pharmacotherapy 2013;47(7‐8):1017‐28. [PUBMED: 23737515] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

