Neural transcription factors: from embryos to neural stem cells
- PMID: 25234468
- PMCID: PMC4213760
- DOI: 10.14348/molcells.2014.0227
Neural transcription factors: from embryos to neural stem cells
Abstract
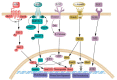
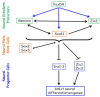
The early steps of neural development in the vertebrate embryo are regulated by sets of transcription factors that control the induction of proliferative, pluripotent neural precursors, the expansion of neural plate stem cells, and their transition to differentiating neural progenitors. These early events are critical for producing a pool of multipotent cells capable of giving rise to the multitude of neurons and glia that form the central nervous system. In this review we summarize findings from gain- and loss-of-function studies in embryos that detail the gene regulatory network responsible for these early events. We discuss whether this information is likely to be similar in mammalian embryonic and induced pluripotent stem cells that are cultured according to protocols designed to produce neurons. The similarities and differences between the embryo and stem cells may provide important guidance to stem cell protocols designed to create immature neural cells for therapeutic uses.
Keywords: Foxd4; neural cell fate; neural induction; neural plate stem cells; neural progenitor cells.
Figures
References
-
- Aruga J., Tohmonda T., Homma S., Mikoshiba K. Zic1 promotes the expansion of dorsal neural progenitors in spinal cord by inhibiting neuronal differentiation. Dev. Biol. 2002;244:329–341. - PubMed
-
- Bani-Yaghoub M., Tremblay R.G., Lei J.X., Zhang D., Zurakowski B., Sandhu J.K., Smith B., Ribecco-Lutkiewicz M., Kennedy J., Walker P.R., et al. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006;295:52–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

