Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome
- PMID: 25234644
- PMCID: PMC4254306
- DOI: 10.1016/j.jaci.2014.07.026
Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome
Abstract
Background: The definition of eosinophilic gastritis (EG) is currently limited to histologic EG based on the tissue eosinophil count.
Objective: We aimed to provide additional fundamental information about the molecular, histopathologic, and clinical characteristics of EG.
Methods: Genome-wide transcript profiles and histologic features of gastric biopsy specimens, as well as blood eosinophil counts, were analyzed in patients with EG and control subjects (n = 15 each).
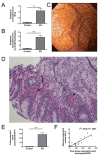
Results: The peak gastric antrum eosinophil count was 283 ± 164 eosinophils/×400 high-power field in patients with EG and 11 ± 9 eosinophils/×400 high-power field in control subjects (P = 6.1 × 10(-7)). Patients with EG (87%) had coexisting eosinophilic inflammation in multiple gastrointestinal segments; the esophagus represented the most common secondary site. Increased peripheral blood eosinophil counts (patients with EG: 1.09 ± 0.88 × 10(3)/μL vs control subjects: 0.09 ± 0.08 10(3)/μL, P = .0027) positively correlated with peak gastric eosinophil counts (Pearson r(2) = .8102, P < .0001). MIB-1(+) (proliferating), CD117(+) (mast cells), and FOXP3(+) (regulatory T cells, activated T cells, or both) cell counts were increased in patients with EG. Transcript profiling revealed changes in 8% of the genome in gastric tissue from patients with EG. Only 7% of this EG transcriptome overlapped with the eosinophilic esophagitis transcriptome. Significantly increased IL4, IL5, IL13, IL17, CCL26, and mast cell-specific transcripts and decreased IL33 transcripts were observed.
Conclusion: EG is a systemic disorder involving profound blood and gastrointestinal tract eosinophilia, TH2 immunity, and a conserved gastric transcriptome markedly distinct from the eosinophilic esophagitis transcriptome. The data herein define germane cellular and molecular pathways of EG and provide a basis for improving diagnosis and treatment.
Keywords: CCL26; EG transcriptome; Eosinophilic gastritis; IL-13; eosinophils; mast cells; regulatory T cells.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


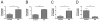
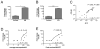
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

