Identification of a novel gene for diabetic traits in rats, mice, and humans
- PMID: 25236446
- PMCID: PMC4174929
- DOI: 10.1534/genetics.114.162982
Identification of a novel gene for diabetic traits in rats, mice, and humans
Abstract
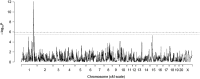
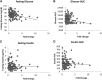
The genetic basis of type 2 diabetes remains incompletely defined despite the use of multiple genetic strategies. Multiparental populations such as heterogeneous stocks (HS) facilitate gene discovery by allowing fine mapping to only a few megabases, significantly decreasing the number of potential candidate genes compared to traditional mapping strategies. In the present work, we employed expression and sequence analysis in HS rats (Rattus norvegicus) to identify Tpcn2 as a likely causal gene underlying a 3.1-Mb locus for glucose and insulin levels. Global gene expression analysis on liver identified Tpcn2 as the only gene in the region that is differentially expressed between HS rats with glucose intolerance and those with normal glucose regulation. Tpcn2 also maps as a cis-regulating expression QTL and is negatively correlated with fasting glucose levels. We used founder sequence to identify variants within this region and assessed association between 18 variants and diabetic traits by conducting a mixed-model analysis, accounting for the complex family structure of the HS. We found that two variants were significantly associated with fasting glucose levels, including a nonsynonymous coding variant within Tpcn2. Studies in Tpcn2 knockout mice demonstrated a significant decrease in fasting glucose levels and insulin response to a glucose challenge relative to those in wild-type mice. Finally, we identified variants within Tpcn2 that are associated with fasting insulin in humans. These studies indicate that Tpcn2 is a likely causal gene that may play a role in human diabetes and demonstrate the utility of multiparental populations for positionally cloning genes within complex loci.
Keywords: MPP; Multiparent Advanced Generation Inter-Cross (MAGIC); Multiparental populations; Tpcn2; expression QTL mapping; glucose; heterogeneous stock rats; insulin; type 2 diabetes.
Copyright © 2014 by the Genetics Society of America.
Figures




References
-
- Aitman T. J., Dong R., Vyse T. J., Norsworthy P. J., Johnson M. D., et al. , 2006. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439(7078): 851–855 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

