A randomized trial of artesunate-amodiaquine versus artemether-lumefantrine in Ghanaian paediatric sickle cell and non-sickle cell disease patients with acute uncomplicated malaria
- PMID: 25236838
- PMCID: PMC4176868
- DOI: 10.1186/1475-2875-13-369
A randomized trial of artesunate-amodiaquine versus artemether-lumefantrine in Ghanaian paediatric sickle cell and non-sickle cell disease patients with acute uncomplicated malaria
Abstract
Background: Sickle cell disease (SCD) is a genetic disorder common in malaria endemic areas. In endemic areas, malaria is a major cause of morbidity and mortality among SCD patients. This suggests the need for prompt initiation of efficacious anti-malarial therapy in SCD patients with acute malaria. However, there is no information to date, on the efficacy or safety of artemisinin combination therapy when used for malaria treatment in SCD patients.
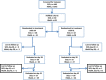
Methods: Children with SCD and acute uncomplicated malaria (n=60) were randomized to treatment with artesunate-amodiaquine (AA), or artemether-lumefantrine (AL). A comparison group of non-SCD children (HbAA genotype; n=59) with uncomplicated malaria were also randomized to treatment with AA or AL. Recruited children were followed up and selected investigations were done on days 1, 2, 3, 7, 14, 28, 35, and 42. Selected clinical and laboratory parameters of the SCD patients were also compared with a group of malaria-negative SCD children (n=82) in steady state.
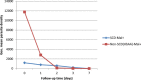
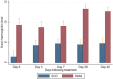
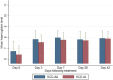
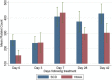
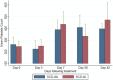
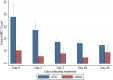
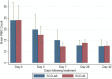
Results: The parasite densities on admission were significantly lower in the SCD group, compared with the non-SCD group (p=0.0006). The parasite reduction ratio (PRR) was lower, clearance was slower (p<0.0001), and time for initial parasitaemia to decline by 50 and 90% were longer for the SCD group. Adequate clinical and parasitological response (ACPR) on day 28 was 98.3% (58/59) in the SCD group and 100% (57/57) in the non-SCD group. Corresponding ACPR rates on day 42 were 96.5% (55/57) in the SCD group and 96.4% (53/55) in the non-SCD group. The fractional changes in haemoglobin, platelets and white blood cell counts between baseline (day 0) and endpoint (day 42) were 16.9, 40.6 and 92.3%, respectively, for the SCD group, and, 12.3, 48.8 and 7.5%, respectively, for the non-SCD group. There were no differences in these indices between AA- and AL-treated subjects.
Conclusions: The parasite clearance of SCD children with uncomplicated malaria was slower compared with non-SCD children. AA and AL showed similar clinical and parasitological effects in the SCD and non-SCD groups. The alterations in WBC and platelet counts may have implications for SCD severity.
Trial registration: Current controlled trials ISRCTN96891086.
Figures

Similar articles
-
Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial.Lancet. 2018 Apr 7;391(10128):1378-1390. doi: 10.1016/S0140-6736(18)30291-5. Epub 2018 Mar 29. Lancet. 2018. PMID: 29606364 Free PMC article. Clinical Trial.
-
Artemether-lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trial.Malar J. 2006 May 16;5:43. doi: 10.1186/1475-2875-5-43. Malar J. 2006. PMID: 16704735 Free PMC article. Clinical Trial.
-
Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up.Malar J. 2008 Jul 11;7:127. doi: 10.1186/1475-2875-7-127. Malar J. 2008. PMID: 18620577 Free PMC article. Clinical Trial.
-
Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD004529. doi: 10.1002/14651858.CD004529.pub3. Cochrane Database Syst Rev. 2021. PMID: 33459345 Free PMC article.
-
Safety and effectiveness of antimalarial therapy in sickle cell disease: a systematic review and network meta-analysis.BMC Infect Dis. 2018 Dec 12;18(1):650. doi: 10.1186/s12879-018-3556-0. BMC Infect Dis. 2018. PMID: 30541465 Free PMC article.
Cited by
-
In vitro delayed response to dihydroartemisinin of malaria parasites infecting sickle cell erythocytes.Malar J. 2024 Jan 4;23(1):9. doi: 10.1186/s12936-023-04819-5. Malar J. 2024. PMID: 38178227 Free PMC article.
-
Artemisinin derivative-containing therapies and abnormal hemoglobin: Do we need to adapt the treatment?Parasite. 2021;28:67. doi: 10.1051/parasite/2021063. Epub 2021 Sep 27. Parasite. 2021. PMID: 34569928 Free PMC article.
-
Suspected Severe Malaria in a Sudanese Patient Affected by Sickle Cell Disease Who Was Treated with Hydroxyurea.Pathogens. 2021 Aug 4;10(8):985. doi: 10.3390/pathogens10080985. Pathogens. 2021. PMID: 34451449 Free PMC article.
-
Population Pharmacokinetic Estimates Suggest Elevated Clearance and Distribution Volume of Desethylamodiaquine in Pediatric Patients with Sickle Cell Disease Treated with Artesunate-Amodiaquine.Curr Ther Res Clin Exp. 2019 Jan 12;90:9-15. doi: 10.1016/j.curtheres.2019.01.005. eCollection 2019. Curr Ther Res Clin Exp. 2019. PMID: 30766619 Free PMC article.
-
Artemisinin Therapy for Malaria in Hemoglobinopathies: A Systematic Review.Clin Infect Dis. 2018 Feb 10;66(5):799-804. doi: 10.1093/cid/cix785. Clin Infect Dis. 2018. PMID: 29370347 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

