Epigenetic events determine tissue-specific toxicity of inhalational exposure to the genotoxic chemical 1,3-butadiene in male C57BL/6J mice
- PMID: 25237060
- PMCID: PMC4250847
- DOI: 10.1093/toxsci/kfu191
Epigenetic events determine tissue-specific toxicity of inhalational exposure to the genotoxic chemical 1,3-butadiene in male C57BL/6J mice
Abstract
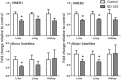
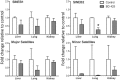
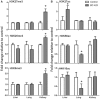
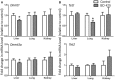
1,3-Butadiene (BD), a widely used industrial chemical and a ubiquitous environmental pollutant, is a known human carcinogen. Although genotoxicity is an established mechanism of the tumorigenicity of BD, epigenetic effects have also been observed in livers of mice exposed to the chemical. To better characterize the diverse molecular mechanisms of BD tumorigenicity, we evaluated genotoxic and epigenotoxic effects of BD exposure in mouse tissues that are target (lung and liver) and non-target (kidney) for BD-induced tumors. We hypothesized that epigenetic alterations may explain, at least in part, the tissue-specific differences in BD tumorigenicity in mice. We evaluated the level of N-7-(2,3,4-trihydroxybut-1-yl)guanine adducts and 1,4-bis-(guan-7-yl)-2,3-butanediol crosslinks, DNA methylation, and histone modifications in male C57BL/6 mice exposed to filtered air or 425 ppm of BD by inhalation (6 h/day, 5 days/week) for 2 weeks. Although DNA damage was observed in all three tissues of BD-exposed mice, variation in epigenetic effects clearly existed between the kidneys, liver, and lungs. Epigenetic alterations indicative of genomic instability, including demethylation of repetitive DNA sequences and alterations in histone-lysine acetylation, were evident in the liver and lung tissues of BD-exposed mice. Changes in DNA methylation were insignificant in the kidneys of treated mice, whereas marks of condensed heterochromatin and transcriptional silencing (histone-lysine trimethylation) were increased. These modifications may represent a potential mechanistic explanation for the lack of tumorigenesis in the kidney. Our results indicate that differential tissue susceptibility to chemical-induced tumorigenesis may be attributed to tissue-specific epigenetic alterations.
Keywords: 1,3-butadiene; epigenetics; genotoxicity.
Published by Oxford University Press on behalf of the Society of Toxicology 2014. This work is written by US Government employees and is in the public domain in the US.
Figures


Similar articles
-
Epigenetic mechanisms of mouse interstrain variability in genotoxicity of the environmental toxicant 1,3-butadiene.Toxicol Sci. 2011 Aug;122(2):448-56. doi: 10.1093/toxsci/kfr133. Epub 2011 May 20. Toxicol Sci. 2011. PMID: 21602187 Free PMC article.
-
Epigenetic alterations in liver of C57BL/6J mice after short-term inhalational exposure to 1,3-butadiene.Environ Health Perspect. 2011 May;119(5):635-40. doi: 10.1289/ehp.1002910. Epub 2010 Dec 13. Environ Health Perspect. 2011. PMID: 21147608 Free PMC article.
-
Population-Based Analysis of DNA Damage and Epigenetic Effects of 1,3-Butadiene in the Mouse.Chem Res Toxicol. 2019 May 20;32(5):887-898. doi: 10.1021/acs.chemrestox.9b00035. Epub 2019 Apr 25. Chem Res Toxicol. 2019. PMID: 30990016
-
A review of the genetic and related effects of 1,3-butadiene in rodents and humans.Mutat Res. 2000 Oct;463(3):181-213. doi: 10.1016/s1383-5742(00)00056-9. Mutat Res. 2000. PMID: 11018742 Review.
-
Epidemiological and mechanistic data suggest that 1,3-butadiene will not be carcinogenic to humans at exposures likely to be encountered in the environment or workplace.Carcinogenesis. 1995 Feb;16(2):165-71. doi: 10.1093/carcin/16.2.165. Carcinogenesis. 1995. PMID: 7859344 Review.
Cited by
-
Environmental pollution and DNA methylation: carcinogenesis, clinical significance, and practical applications.Front Med. 2015 Sep;9(3):261-74. doi: 10.1007/s11684-015-0406-y. Epub 2015 Aug 19. Front Med. 2015. PMID: 26290283 Review.
-
Tissue- and strain-specific effects of a genotoxic carcinogen 1,3-butadiene on chromatin and transcription.Mamm Genome. 2018 Feb;29(1-2):153-167. doi: 10.1007/s00335-018-9739-6. Epub 2018 Feb 10. Mamm Genome. 2018. PMID: 29429127 Free PMC article.
-
An Assessment on Ethanol-Blended Gasoline/Diesel Fuels on Cancer Risk and Mortality.Int J Environ Res Public Health. 2021 Jun 28;18(13):6930. doi: 10.3390/ijerph18136930. Int J Environ Res Public Health. 2021. PMID: 34203568 Free PMC article. Review.
-
Effects of continuous bisphenol A exposure from early gestation on 90 day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study.Toxicol Appl Pharmacol. 2018 May 15;347:1-9. doi: 10.1016/j.taap.2018.03.021. Epub 2018 Mar 26. Toxicol Appl Pharmacol. 2018. PMID: 29596923 Free PMC article.
-
Characterization of population variability of 1,3-butadiene derived protein adducts in humans and mice.Regul Toxicol Pharmacol. 2022 Jul;132:105171. doi: 10.1016/j.yrtph.2022.105171. Epub 2022 Apr 22. Regul Toxicol Pharmacol. 2022. PMID: 35469930 Free PMC article.
References
-
- Bond J. A., Medinsky M. A. (2001). Insights into the toxicokinetics and toxicodynamics of 1,3-butadiene. Chem. Biol. Interact. 135–136, 599–614. - PubMed
-
- Delzell E., Sathiakumar N., Hovinga M., Macaluso M., Julian J., Larson R., Cole P., Muir D. C. (1996). A follow-up study of synthetic rubber workers. Toxicology 113, 182–189. - PubMed
-
- Dillon N. (2004). Heterochromatin structure and function. Biol. Cell 96, 631–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

