Pharmacologic considerations in use and development of antituberculosis drugs
- PMID: 25237145
- PMCID: PMC4292083
- DOI: 10.1101/cshperspect.a021170
Pharmacologic considerations in use and development of antituberculosis drugs
Abstract
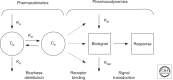
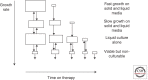
Rational development and deployment of antituberculosis drugs depend on a comprehensive understanding of the pharmacokinetics and pharmacodynamics that underlie their clinical behavior. Successful implementation of a pharmacokinetic-pharmacodynamic approach faces difficulties that, although not unique to tuberculosis as a therapeutic area, in combination pose a significant scientific challenge. In recent years, a multidisciplinary response combining new technological and analytical approaches has begun to directly address many of these issues, shedding light on some previously poorly understood aspects of drug distribution and response. These advances have important implications for optimization of existing and development of novel drug regimens, putting quantitative pharmacology at the heart of preclinical and early drug development.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future.Antimicrob Agents Chemother. 2011 Jan;55(1):24-34. doi: 10.1128/AAC.00749-10. Epub 2010 Oct 11. Antimicrob Agents Chemother. 2011. PMID: 20937778 Free PMC article. Review.
-
Preclinical Efficacy Testing of New Drug Candidates.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.tbtb2-0034-2017. doi: 10.1128/microbiolspec.TBTB2-0034-2017. Microbiol Spectr. 2017. PMID: 28643624 Free PMC article. Review.
-
Biopharmaceutics, pharmacokinetics and pharmacodynamics of antituberculosis drugs.Curr Med Chem. 2008;15(8):809-25. doi: 10.2174/092986708783955509. Curr Med Chem. 2008. PMID: 18393850 Review.
-
Pharmacokinetics and pharmacodynamics in the development of anti-tuberculosis drugs.Tuberculosis (Edinb). 2008 Aug;88 Suppl 1:S65-74. doi: 10.1016/S1472-9792(08)70037-4. Tuberculosis (Edinb). 2008. PMID: 18762154 Review.
-
Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia.J Infect Dis. 2015 Jun 15;211 Suppl 3:S96-S106. doi: 10.1093/infdis/jiu610. J Infect Dis. 2015. PMID: 26009618 Review.
Cited by
-
Microfluidic dose-response platform to track the dynamics of drug response in single mycobacterial cells.Sci Rep. 2022 Nov 15;12(1):19578. doi: 10.1038/s41598-022-24175-9. Sci Rep. 2022. PMID: 36379978 Free PMC article.
References
-
- Burhan E, Ruesen C, Ruslami R, Ginanjar A, Mangunnegoro H, Ascobat P, Donders R, van Crevel R, Aarnoutse R 2013. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother 57: 3614–3619. - PMC - PubMed
-
- Canetti G 1962. The eradication of tuberculosis: Theoretical problems and practical solutions. Tubercle 43: 301–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
