Differential contribution of TRPM4 and TRPM5 nonselective cation channels to the slow afterdepolarization in mouse prefrontal cortex neurons
- PMID: 25237295
- PMCID: PMC4154465
- DOI: 10.3389/fncel.2014.00267
Differential contribution of TRPM4 and TRPM5 nonselective cation channels to the slow afterdepolarization in mouse prefrontal cortex neurons
Abstract
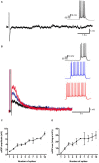
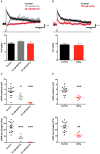
In certain neurons from different brain regions, a brief burst of action potentials can activate a slow afterdepolarization (sADP) in the presence of muscarinic acetylcholine receptor agonists. The sADP, if suprathreshold, can contribute to persistent non-accommodating firing in some of these neurons. Previous studies have characterized a Ca(2+)-activated non-selective cation (CAN) current (ICAN ) that is thought to underlie the sADP. ICAN depends on muscarinic receptor stimulation and exhibits a dependence on neuronal activity, membrane depolarization and Ca(2+)-influx similar to that observed for the sADP. Despite the widespread occurrence of sADPs in neurons throughout the brain, the molecular identity of the ion channels underlying these events, as well as ICAN , remains uncertain. Here we used a combination of genetic, pharmacological and electrophysiological approaches to characterize the molecular mechanisms underlying the muscarinic receptor-dependent sADP in layer 5 pyramidal neurons of mouse prefrontal cortex. First, we confirmed that in the presence of the cholinergic agonist carbachol a brief burst of action potentials triggers a prominent sADP in these neurons. Second, we confirmed that this sADP requires activation of a PLC signaling cascade and intracellular calcium signaling. Third, we obtained direct evidence that the transient receptor potential (TRP) melastatin 5 channel (TRPM5), which is thought to function as a CAN channel in non-neural cells, contributes importantly to the sADP in the layer 5 neurons. In contrast, the closely related TRPM4 channel may play only a minor role in the sADP.
Keywords: Ca2+-activated non-selective cation (CAN) current; muscarinic receptors; persistent firing; slow afterdepolarization (sADP); transient receptor potential melastatin 5 channel (TRPM5).
Figures




Similar articles
-
Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex.J Neurophysiol. 1998 Sep;80(3):1197-210. doi: 10.1152/jn.1998.80.3.1197. J Neurophysiol. 1998. PMID: 9744932
-
ICAN (TRPM4) Contributes to the Intrinsic Excitability of Prefrontal Cortex Layer 2/3 Pyramidal Neurons.Int J Mol Sci. 2021 May 17;22(10):5268. doi: 10.3390/ijms22105268. Int J Mol Sci. 2021. PMID: 34067824 Free PMC article.
-
Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility.J Neurosci. 2006 Nov 15;26(46):11961-73. doi: 10.1523/JNEUROSCI.3171-06.2006. J Neurosci. 2006. PMID: 17108170 Free PMC article.
-
The non-selective monovalent cationic channels TRPM4 and TRPM5.Adv Exp Med Biol. 2011;704:147-71. doi: 10.1007/978-94-007-0265-3_8. Adv Exp Med Biol. 2011. PMID: 21290294 Review.
-
The Ca2+-Activated TRP Channels: TRPM4 and TRPM5.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 15. PMID: 21204504 Free Books & Documents. Review.
Cited by
-
Long-Lasting Sound-Evoked Afterdischarge in the Auditory Midbrain.Sci Rep. 2016 Feb 12;6:20757. doi: 10.1038/srep20757. Sci Rep. 2016. PMID: 26867811 Free PMC article.
-
TRPM4 mediates a subthreshold membrane potential oscillation in respiratory chemoreceptor neurons that drives pacemaker firing and breathing.Cell Rep. 2021 Feb 2;34(5):108714. doi: 10.1016/j.celrep.2021.108714. Cell Rep. 2021. PMID: 33535052 Free PMC article.
-
Cell-type-specific cholinergic control of granular retrosplenial cortex with implications for angular velocity coding across brain states.Prog Neurobiol. 2025 Aug;251:102804. doi: 10.1016/j.pneurobio.2025.102804. Epub 2025 Jul 8. Prog Neurobiol. 2025. PMID: 40639485 Free PMC article.
-
A unifying hypothesis for M1 muscarinic receptor signalling in pyramidal neurons.J Physiol. 2017 Mar 1;595(5):1711-1723. doi: 10.1113/JP273627. Epub 2016 Dec 17. J Physiol. 2017. PMID: 27861914 Free PMC article.
-
Short-term Hebbian learning can implement transformer-like attention.PLoS Comput Biol. 2024 Jan 26;20(1):e1011843. doi: 10.1371/journal.pcbi.1011843. eCollection 2024 Jan. PLoS Comput Biol. 2024. PMID: 38277432 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

