Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study
- PMID: 25237400
- PMCID: PMC4167135
- DOI: 10.1186/1758-5996-6-95
Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study
Abstract
Background: Liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, has been shown to possess pleiotropic effects including body weight reduction. However, long-term effect of liraglutide on body weight and glycemic control has not been elucidated in Japanese type 2 diabetes (T2D) subjects. Present study investigates whether liraglutide treatment maintains the body weight-decreasing and glucose-lowering effects for 2 years in Japanese T2D subjects.
Methods: The enrolled subjects were 86 T2D patients [age; 59.8 ± 12.8 years, duration of diabetes; 15.8 ± 9.5 years, glycated hemoglobin (HbA1c); 8.5 ± 1.5%, body mass index (BMI); 27.3 ± 5.4 kg/m(2) (15.8 - 46.5 kg/m(2)), mean ± SD]. Among 86 subjects, liraglutide was introduced in 25 inpatients and 61 outpatients, and 46 subjects were followed for 2 years. Clinical parameters were measured at baseline and 3, 6, 9, 12, and 24 months after liraglutide introduction. The increase in liraglutide dosage and the additional usage of glucose-lowering agents depended on each attending physician.
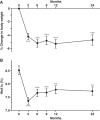
Results: At 1 year after liraglutide introduction, 69 patients (80.2%) decreased body weight and 58 patients (67.4%) improved glycemic control. Body mass index (BMI) was changed 27.3 ± 5.4 kg/m(2) to 25.9 ± 4.8 kg/m(2) and percent reduction of body weight was significant and maintained over 4% at 2 years after liraglutide introduction. HbA1c was significantly decreased from 8.5 ± 1.5% to 7.7 ± 1.2% for 2 years. Liraglutide treatment tended to ameliorate lipid profile and hepatic enzymes. Stepwise regression analysis demonstrated that baseline BMI and previous insulin dose were positively associated with body weight reduction and baseline HbA1c was positively associated with reduction of HbA1c at 2 years after liraglutide introduction.
Conclusions: Long-term liraglutide treatment effectively maintained the reduction of body weight and the fair glycemic control, and also improved lipid profile and liver enzymes in Japanese T2D subjects.
Keywords: Diabetes; Eating behavior; Glucagon-like peptide-1; Liraglutide; Metabolic syndrome; Obesity.
Figures



Similar articles
-
Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes.Cardiovasc Diabetol. 2012 Sep 14;11:107. doi: 10.1186/1475-2840-11-107. Cardiovasc Diabetol. 2012. PMID: 22973968 Free PMC article.
-
Long-Term Effectiveness of Liraglutide in Association with Patients' Baseline Characteristics in Real-Life Setting in Croatia: An Observational, Retrospective, Multicenter Study.Diabetes Ther. 2017 Dec;8(6):1297-1308. doi: 10.1007/s13300-017-0324-x. Epub 2017 Oct 26. Diabetes Ther. 2017. PMID: 29076038 Free PMC article.
-
Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes.Cardiovasc Diabetol. 2011 Dec 1;10:109. doi: 10.1186/1475-2840-10-109. Cardiovasc Diabetol. 2011. PMID: 22132774 Free PMC article.
-
First fixed-ratio combination of insulin degludec and liraglutide for the treatment of type 2 diabetes.Drugs Today (Barc). 2015 Mar;51(3):185-96. doi: 10.1358/dot.2015.51.3.2294596. Drugs Today (Barc). 2015. PMID: 25876562 Review.
-
The Role of the Pharmacist in Managing Type 2 Diabetes with Glucagon-Like Peptide-1 Receptor Agonists as Add-On Therapy.Adv Ther. 2017 Mar;34(3):638-657. doi: 10.1007/s12325-017-0491-1. Epub 2017 Feb 16. Adv Ther. 2017. PMID: 28210986 Review.
Cited by
-
Real-world efficacy and safety of naltrexone-bupropion therapy in Chinese patients with obesity: A single-centre experience.Endocrine. 2025 Feb;87(2):522-529. doi: 10.1007/s12020-024-04029-2. Epub 2024 Oct 5. Endocrine. 2025. PMID: 39367996 Free PMC article.
-
Dipeptidyl peptidase-4 inhibitors as preferable oral hypoglycemic agents in terms of treatment satisfaction: Results from a multicenter, 12-week, open label, randomized controlled study in Japan (PREFERENCE 4 study).J Diabetes Investig. 2018 Jan;9(1):137-145. doi: 10.1111/jdi.12659. Epub 2017 May 2. J Diabetes Investig. 2018. PMID: 28296349 Free PMC article. Clinical Trial.
-
Clinical Effectiveness of Liraglutide in Type 2 Diabetes Treatment in the Real-World Setting: A Systematic Literature Review.Diabetes Ther. 2016 Sep;7(3):411-38. doi: 10.1007/s13300-016-0180-0. Epub 2016 Jun 27. Diabetes Ther. 2016. PMID: 27350545 Free PMC article. Review.
-
Effectiveness and Persistence of Liraglutide Treatment Among Patients with Type 2 Diabetes Treated in Primary Care and Specialist Settings: A Subgroup Analysis from the EVIDENCE Study, a Prospective, 2-Year Follow-up, Observational, Post-Marketing Study.Adv Ther. 2017 Mar;34(3):674-685. doi: 10.1007/s12325-017-0476-0. Epub 2017 Jan 30. Adv Ther. 2017. PMID: 28138803 Free PMC article.
-
Hypothalamic Obesity in Craniopharyngioma Patients: Disturbed Energy Homeostasis Related to Extent of Hypothalamic Damage and Its Implication for Obesity Intervention.J Clin Med. 2015 Sep 9;4(9):1774-97. doi: 10.3390/jcm4091774. J Clin Med. 2015. PMID: 26371051 Free PMC article. Review.
References
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

