Nonresponders to daily paroxetine and another SSRI in men with lifelong premature ejaculation: a pharmacokinetic dose-escalation study for a rare phenomenon
- PMID: 25237462
- PMCID: PMC4165923
- DOI: 10.4111/kju.2014.55.9.599
Nonresponders to daily paroxetine and another SSRI in men with lifelong premature ejaculation: a pharmacokinetic dose-escalation study for a rare phenomenon
Abstract
Purpose: Nonresponse to any selective serotonin reuptake inhibitor (SSRI) treatment is rare. In this study, we aimed to investigate ejaculation delay nonresponse to paroxetine treatment in men with lifelong premature ejaculation (PE) who were also known to be nonresponders to other SSRIs.
Materials and methods: Five males with lifelong PE who were known nonresponders to paroxetine and other serotonergic antidepressants and eight males with lifelong PE who were specifically recruited were included. Blood sampling occurred 1 month and 1 day before the start of treatment and at the end of three consecutive series of 4 weeks of daily treatment with 10-, 20-, and 30-mg paroxetine, respectively. Blood samples for measurement of leptin and paroxetine were taken at 8:30 AM, 9:30 AM, 10:30 AM, and 11:30 AM, respectively. At 9:00 AM, one tablet of 10-, 20-, or 30-mg paroxetine was taken during the first, second, and third month, respectively. Intravaginal ejaculatory latency time (IELT) was measured with a stopwatch. The main outcome measures were the fold increase in the geometric mean IELT, serum leptin and paroxetine concentrations, body mass index (BMI), 5-HT1A receptor C-1019G polymorphism, and CYP2D6 mutations.
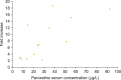
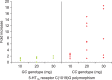
Results: Between the 7 paroxetine responders and 6 nonresponders, the fold increase in the geometric mean IELT was significantly different after daily 10-mg (p=0.003), 20-mg (p=0.002), and 30-mg paroxetine (p=0.026) and ranged from 2.0 to 8.8 and from 1.1 to 1.7, respectively. BMI at baseline and at the end of the study was not significantly different between responders and nonresponders. Serum leptin levels at baseline were similar in responders and nonresponders and did not change during treatment. The serum paroxetine concentration increased with increasing dosage and was not significantly different between responders and nonresponders. There was no association between the fold increase in the geometric mean IELT and serum paroxetine levels during the three treatment periods nor between leptin levels during the treatment periods and serum paroxetine levels. For the 5-HT1A receptor C-1019G variation, all responders had the CC genotype and all nonresponders had the GC genotype, respectively.
Conclusions: Complete absence of paroxetine-induced ejaculation delay is presumably related to pharmacodynamic factors and perhaps to 5-HT1A receptor gene polymorphism.
Keywords: 5-HT1A (C-1019G) polymorphism; CYP2D6; Leptin; Lifelong premature ejaculation; Paroxetine hemihydrate.
Conflict of interest statement
The authors have nothing to disclose.
Figures

Similar articles
-
Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline.J Clin Psychopharmacol. 1998 Aug;18(4):274-81. doi: 10.1097/00004714-199808000-00004. J Clin Psychopharmacol. 1998. PMID: 9690692 Clinical Trial.
-
Relevance of serum nitric oxide levels and the efficacy of selective serotonin reuptake inhibitors treatment on premature ejaculation: decreased nitric oxide is associated with premature ejaculation.Andrologia. 2014;46(9):951-5. doi: 10.1111/and.12179. Epub 2013 Oct 11. Andrologia. 2014. PMID: 24118023 Clinical Trial.
-
Effects of paroxetine on intravaginal ejaculatory latency time in Egyptian patients with lifelong premature ejaculation as a function of serotonin transporter polymorphism.Int J Impot Res. 2017 Jan;29(1):7-11. doi: 10.1038/ijir.2016.36. Epub 2016 Sep 29. Int J Impot Res. 2017. PMID: 27679962 Clinical Trial.
-
Pharmacotherapy for premature ejaculation.Expert Opin Pharmacother. 2015;16(17):2615-24. doi: 10.1517/14656566.2015.1096928. Epub 2015 Nov 18. Expert Opin Pharmacother. 2015. PMID: 26579971 Review.
-
Emerging drugs for premature ejaculation.Expert Opin Emerg Drugs. 2006 Mar;11(1):99-109. doi: 10.1517/14728214.11.1.99. Expert Opin Emerg Drugs. 2006. PMID: 16503829 Review.
Cited by
-
Possible Pathophysiological Roles of Neurotransmitter Systems in Men With Lifelong Premature Ejaculation: Protocol for a Scoping Review.JMIR Res Protoc. 2023 Mar 13;12:e41301. doi: 10.2196/41301. JMIR Res Protoc. 2023. PMID: 36912871 Free PMC article.
-
The office management of ejaculatory disorders.Transl Androl Urol. 2016 Aug;5(4):526-40. doi: 10.21037/tau.2016.05.07. Transl Androl Urol. 2016. PMID: 27652225 Free PMC article. Review.
References
-
- Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res. 2004;16:369–381. - PubMed
-
- Salonia A, Rocchini L, Sacca' A, Pellucchi F, Ferrari M, Carro UD, et al. Acceptance of and discontinuation rate from paroxetine treatment in patients with lifelong premature ejaculation. J Sex Med. 2009;6:2868–2877. - PubMed
-
- Sindrup SH, Brosen K, Gram LF, Hallas J, Skjelbo E, Allen A, et al. The relationship between paroxetine and the sparteine oxidation polymorphism. Clin Pharmacol Ther. 1992;51:278–287. - PubMed
-
- de Jong TR, Pattij T, Veening JG, Waldinger MD, Cools AR, Olivier B. Effects of chronic selective serotonin reuptake inhibitors on 8-OH-DPAT-induced facilitation of ejaculation in rats: comparison of fluvoxamine and paroxetine. Psychopharmacology (Berl) 2005;179:509–515. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

