Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis
- PMID: 25237811
- PMCID: PMC4169583
- DOI: 10.1371/journal.pone.0108270
Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis
Abstract
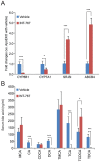
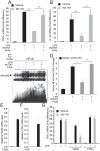
Bile acid signaling is a critical regulator of glucose and energy metabolism, mainly through the nuclear receptor FXR and the G protein-coupled receptor TGR. The purpose of the present study was to investigate whether dual activation of FXR and TGR5 plays a significant role in the prevention of atherosclerosis progression. To evaluate the effects of bile acid signaling in atherogenesis, ApoE-/- mice and LDLR-/- mice were treated with an FXR/TGR5 dual agonist (INT-767). INT-767 treatment drastically reduced serum cholesterol levels. INT-767 treatment significantly reduced atherosclerotic plaque formation in both ApoE-/- and LDLR-/- mice. INT-767 decreased the expression of pro-inflammatory cytokines and chemokines in the aortas of ApoE-/- mice through the inactivation of NF-κB. In addition, J774 macrophages treated with INT-767 had significantly lower levels of active NF-κB, resulting in cytokine production in response to LPS through a PKA dependent mechanism. This study demonstrates that concurrent activation of FXR and TGR5 attenuates atherosclerosis by reducing both circulating lipids and inflammation.
Conflict of interest statement
Figures




Similar articles
-
Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation.J Lipid Res. 2018 Sep;59(9):1709-1713. doi: 10.1194/jlr.M087239. Epub 2018 Jul 5. J Lipid Res. 2018. PMID: 29976576 Free PMC article.
-
FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity.J Am Soc Nephrol. 2018 Jan;29(1):118-137. doi: 10.1681/ASN.2017020222. Epub 2017 Oct 31. J Am Soc Nephrol. 2018. PMID: 29089371 Free PMC article.
-
A dual agonist of farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5, INT-767, reverses age-related kidney disease in mice.J Biol Chem. 2017 Jul 21;292(29):12018-12024. doi: 10.1074/jbc.C117.794982. Epub 2017 Jun 8. J Biol Chem. 2017. PMID: 28596381 Free PMC article.
-
A role of the bile salt receptor FXR in atherosclerosis.Arterioscler Thromb Vasc Biol. 2010 Aug;30(8):1519-28. doi: 10.1161/ATVBAHA.109.197897. Arterioscler Thromb Vasc Biol. 2010. PMID: 20631352 Review.
-
Bile acid receptors in non-alcoholic fatty liver disease.Biochem Pharmacol. 2013 Dec 1;86(11):1517-24. doi: 10.1016/j.bcp.2013.08.015. Epub 2013 Aug 26. Biochem Pharmacol. 2013. PMID: 23988487 Free PMC article. Review.
Cited by
-
The role of the gut microbiota in health and cardiovascular diseases.Mol Biomed. 2022 Oct 11;3(1):30. doi: 10.1186/s43556-022-00091-2. Mol Biomed. 2022. PMID: 36219347 Free PMC article. Review.
-
Bile acid receptors link nutrient sensing to metabolic regulation.Liver Res. 2017 Jun;1(1):17-25. doi: 10.1016/j.livres.2017.04.001. Epub 2017 Apr 26. Liver Res. 2017. PMID: 29098111 Free PMC article.
-
The Role of Gut Microbiota in Atherosclerosis and Hypertension.Front Pharmacol. 2018 Sep 25;9:1082. doi: 10.3389/fphar.2018.01082. eCollection 2018. Front Pharmacol. 2018. PMID: 30319417 Free PMC article. Review.
-
Bile Acid Network and Vascular Calcification-Associated Diseases: Unraveling the Intricate Connections and Therapeutic Potential.Clin Interv Aging. 2023 Oct 21;18:1749-1767. doi: 10.2147/CIA.S431220. eCollection 2023. Clin Interv Aging. 2023. PMID: 37885621 Free PMC article. Review.
-
Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation.J Lipid Res. 2018 Sep;59(9):1709-1713. doi: 10.1194/jlr.M087239. Epub 2018 Jul 5. J Lipid Res. 2018. PMID: 29976576 Free PMC article.
References
-
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, et al. (1999) Identification of a nuclear receptor for bile acids. Science 284: 1362–1365. - PubMed
-
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368. - PubMed
-
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553. - PubMed
-
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y (2006) FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31: 572–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

