Chemical biology strategies for posttranslational control of protein function
- PMID: 25237866
- PMCID: PMC4174368
- DOI: 10.1016/j.chembiol.2014.08.011
Chemical biology strategies for posttranslational control of protein function
Abstract
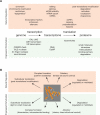
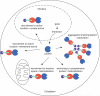
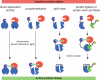
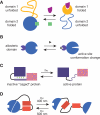
A common strategy to understand a biological system is to selectively perturb it and observe its response. Although technologies now exist to manipulate cellular systems at the genetic and transcript level, the direct manipulation of functions at the protein level can offer significant advantages in precision, speed, and reversibility. Combining the specificity of genetic manipulation and the spatiotemporal resolution of light- and small molecule-based approaches now allows exquisite control over biological systems to subtly perturb a system of interest in vitro and in vivo. Conditional perturbation mechanisms may be broadly characterized by change in intracellular localization, intramolecular activation, or degradation of a protein-of-interest. Here we review recent advances in technologies for conditional regulation of protein function and suggest further areas of potential development.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Armstrong CM, Goldberg DE. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat. Methods. 2007;4:1007–1009. - PubMed
-
- Banaszynski LA, Wandless TJ. Conditional control of protein function. Chem. Biol. 2006;13:11–21. - PubMed
-
- Banaszynski LA, Liu CW, Wandless TJ. Characterization of the FKBP rapamycin FRB ternary complex. J. Am. Chem. Soc. 2005;127:4715–4721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

