Arsenite-activated JNK signaling enhances CPEB4-Vinexin interaction to facilitate stress granule assembly and cell survival
- PMID: 25237887
- PMCID: PMC4169592
- DOI: 10.1371/journal.pone.0107961
Arsenite-activated JNK signaling enhances CPEB4-Vinexin interaction to facilitate stress granule assembly and cell survival
Abstract
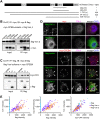
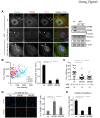
Stress granules (SGs) are compartmentalized messenger ribonucleoprotein particles (mRNPs) where translationally repressed mRNAs are stored when cells encounter environmental stress. Cytoplasmic polyadenylation element-binding protein (CPEB)4 is a sequence-specific RNA-binding protein and translational regulator. In keeping with the results obtained from the study of other RNA-binding proteins, we found CPEB4 localized in SGs in various arsenite-treated cells. In this study, we identified that Vinexin, a CPEB4-interacting protein, is a novel component of SGs. Vinexin is a SH3-domain-containing adaptor protein and affects cell migration through its association with Vinculin to localize at focal adhesions (FAs). Unexpectedly, Vinexin is translocated from FAs to SGs under arsenite-induced stress. The recruitment of Vinexin to SGs depends on its interaction with CPEB4 and influences SG formation and cell survival. Arsenite-activated c-Jun N-terminal kinase (JNK) signaling enhances the association between CPEB4 and Vinexin, which consequently facilitates SG localization of Vinexin. Taken together, this study uncovers a novel interaction between a translational regulator and an adaptor protein to influence SG assembly and cell survival.
Conflict of interest statement
Figures





Similar articles
-
Phosphorylation of Tudor-SN, a novel substrate of JNK, is involved in the efficient recruitment of Tudor-SN into stress granules.Biochim Biophys Acta Mol Cell Res. 2017 Mar;1864(3):562-571. doi: 10.1016/j.bbamcr.2016.12.018. Epub 2016 Dec 21. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28011284
-
Interaction of a multi-domain adaptor protein, vinexin, with a Rho-effector, Rhotekin.Med Mol Morphol. 2009 Mar;42(1):9-15. doi: 10.1007/s00795-008-0433-8. Epub 2009 Mar 18. Med Mol Morphol. 2009. PMID: 19294487
-
Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly.J Biol Chem. 2016 Oct 21;291(43):22671-22685. doi: 10.1074/jbc.M116.739573. Epub 2016 Sep 6. J Biol Chem. 2016. PMID: 27601476 Free PMC article.
-
Changing faces of stress: Impact of heat and arsenite treatment on the composition of stress granules.Wiley Interdiscip Rev RNA. 2020 Nov;11(6):e1596. doi: 10.1002/wrna.1596. Epub 2020 May 3. Wiley Interdiscip Rev RNA. 2020. PMID: 32362075 Review.
-
[The Molecular Basis of Drug Discovery Targeting the Regulatory Mechanism of MAPK Signaling via the Spatial Regulation of RNA-binding Proteins].Yakugaku Zasshi. 2019;139(1):7-12. doi: 10.1248/yakushi.18-00189. Yakugaku Zasshi. 2019. PMID: 30606933 Review. Japanese.
Cited by
-
Who Regulates Whom? An Overview of RNA Granules and Viral Infections.Viruses. 2016 Jun 28;8(7):180. doi: 10.3390/v8070180. Viruses. 2016. PMID: 27367717 Free PMC article. Review.
-
Sorbin and SH3 domain-containing protein 2 (SORBS2) is a component of the acto-myosin ring at the apical junctional complex in epithelial cells.PLoS One. 2017 Sep 29;12(9):e0185448. doi: 10.1371/journal.pone.0185448. eCollection 2017. PLoS One. 2017. PMID: 28961272 Free PMC article.
-
Methyltransferase 3 Mediated miRNA m6A Methylation Promotes Stress Granule Formation in the Early Stage of Acute Ischemic Stroke.Front Mol Neurosci. 2020 Jun 5;13:103. doi: 10.3389/fnmol.2020.00103. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32581712 Free PMC article.
-
CPEB4 is regulated during cell cycle by ERK2/Cdk1-mediated phosphorylation and its assembly into liquid-like droplets.Elife. 2016 Nov 1;5:e19298. doi: 10.7554/eLife.19298. Elife. 2016. PMID: 27802129 Free PMC article.
-
Biphasic and Stage-Associated Expression of CPEB4 in Hepatocellular Carcinoma.PLoS One. 2016 May 9;11(5):e0155025. doi: 10.1371/journal.pone.0155025. eCollection 2016. PLoS One. 2016. PMID: 27158894 Free PMC article.
References
-
- Anderson P, Kedersha N (2009) RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 10: 430–436. - PubMed
-
- Higashi S, Kabuta T, Nagai Y, Tsuchiya Y, Akiyama H, et al. (2013) TDP-43 associates with stalled ribosomes and contributes to cell survival during cellular stress. J Neurochem 126: 288–300. - PubMed
-
- Kim B, Cooke HJ, Rhee K (2012) DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 139: 568–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

