Phenylalanine in the pore of the Erwinia ligand-gated ion channel modulates picrotoxinin potency but not receptor function
- PMID: 25238029
- PMCID: PMC4312132
- DOI: 10.1021/bi5008035
Phenylalanine in the pore of the Erwinia ligand-gated ion channel modulates picrotoxinin potency but not receptor function
Abstract
The Erwinia ligand-gated ion channel (ELIC) is a bacterial homologue of eukaryotic Cys-loop ligand-gated ion channels. This protein has the potential to be a useful model for Cys-loop receptors but is unusual in that it has an aromatic residue (Phe) facing into the pore, leading to some predictions that this protein is incapable of ion flux. Subsequent studies have shown this is not the case, so here we probe the role of this residue by examining the function of the ELIC in cases in which the Phe has been substituted with a range of alternative amino acids, expressed in Xenopus oocytes and functionally examined. Most of the mutations have little effect on the GABA EC50, but the potency of the weak pore-blocking antagonist picrotoxinin at F16'A-, F16'D-, F16'S-, and F16'T-containing receptors was increased to levels comparable with those of Cys-loop receptors, suggesting that this antagonist can enter the pore only when residue 16' is small. T6'S has no effect on picrotoxinin potency when expressed alone but abolishes the increased potency when combined with F16'S, indicating that the inhibitor binds at position 6', as in Cys-loop receptors, if it can enter the pore. Overall, the data support the proposal that the ELIC pore is a good model for Cys-loop receptor pores if the role of F16' is taken into consideration.
Figures


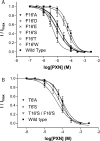
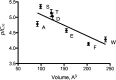


Similar articles
-
The pharmacological profile of ELIC, a prokaryotic GABA-gated receptor.Neuropharmacology. 2012 Sep;63(4):761-7. doi: 10.1016/j.neuropharm.2012.05.027. Epub 2012 Jun 4. Neuropharmacology. 2012. PMID: 22677470 Free PMC article.
-
Allosteric binding site in a Cys-loop receptor ligand-binding domain unveiled in the crystal structure of ELIC in complex with chlorpromazine.Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):E6696-E6703. doi: 10.1073/pnas.1603101113. Epub 2016 Oct 10. Proc Natl Acad Sci U S A. 2016. PMID: 27791038 Free PMC article.
-
Cys-loop receptor channel blockers also block GLIC.Biophys J. 2011 Dec 21;101(12):2912-8. doi: 10.1016/j.bpj.2011.10.055. Epub 2011 Dec 20. Biophys J. 2011. PMID: 22208189 Free PMC article.
-
Structural insights into Cys-loop receptor function and ligand recognition.Biochem Pharmacol. 2013 Oct 15;86(8):1042-53. doi: 10.1016/j.bcp.2013.07.001. Epub 2013 Jul 10. Biochem Pharmacol. 2013. PMID: 23850718 Review.
-
The structural basis of function in Cys-loop receptors.Q Rev Biophys. 2010 Nov;43(4):449-99. doi: 10.1017/S0033583510000168. Epub 2010 Sep 20. Q Rev Biophys. 2010. PMID: 20849671 Review.
Cited by
-
Common Anesthetic-binding Site for Inhibition of Pentameric Ligand-gated Ion Channels.Anesthesiology. 2016 Mar;124(3):664-73. doi: 10.1097/ALN.0000000000001005. Anesthesiology. 2016. PMID: 26756520 Free PMC article.
References
-
- Bocquet N.; Nury H.; Baaden M.; Le Poupon C.; Changeux J. P.; Delarue M.; Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114. - PubMed
-
- Hilf R. J.; Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118. - PubMed
-
- Hilf R. J.; Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379. - PubMed
-
- Spurny R.; Ramerstorfer J.; Price K.; Brams M.; Ernst M.; Nury H.; Verheij M.; Legrand P.; Bertrand D.; Bertrand S.; Dougherty D. A.; de Esch I. J.; Corringer P. J.; Sieghart W.; Lummis S. C.; Ulens C. (2012) Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc. Natl. Acad. Sci. U.S.A. 109, E3028–E3034. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

