Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat--and with which drug--those are the questions
- PMID: 25238206
- PMCID: PMC4255127
- DOI: 10.1210/jc.2014-2811
Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat--and with which drug--those are the questions
Abstract
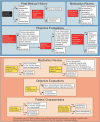
Context: Medullary thyroid cancer (MTC) is a rare form of thyroid cancer comprising approximately 4% of all thyroid cancers. The majority of patients have a relatively good prognosis; however, a subgroup of patients will require systemic therapy. Large phase III randomized trials led to the approval of two drugs--vandetanib and cabozantinib--for progressive or symptomatic MTC. The decision regarding which drug to initiate first is not entirely clear and is a common concern amongst treating physicians.
Evidence acquisition and synthesis: A review of the literature in English was conducted, and data were summarized and integrated into a decision matrix.
Conclusions: The decision regarding which drug to initiate first for progressive MTC should be based on a careful review of the medical history, physical examination findings, medication list, electrocardiogram, laboratory results, and tumor characteristics. It is necessary to consider the relative contraindications when choosing which drug to initiate first.
Figures
References
-
- Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. - PubMed
-
- Boostrom SY, Grant CS, Thompson GB, et al. Need for a revised staging consensus in medullary thyroid carcinoma. Arch Surg. 2009;144:663–669. - PubMed
-
- Meijer JA, le Cessie S, van den Hout WB, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf). 2010;72:534–542. - PubMed
-
- Calcitonin and carcinoembryonic antigen (CEA) doubling time calculator. American Thyroid Association. http://www.thyroid.org/thyroid-physicians-professionals/calculators/thyr... Accessed August 11, 2014.
-
- Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005;90:2029–2034. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

