Intravenous immunoglobulin for Guillain-Barré syndrome
- PMID: 25238327
- PMCID: PMC6781841
- DOI: 10.1002/14651858.CD002063.pub6
Intravenous immunoglobulin for Guillain-Barré syndrome
Abstract
Background: Guillain-Barré syndrome (GBS) is an acute, paralysing, inflammatory peripheral nerve disease. Intravenous immunoglobulin (IVIg) is beneficial in other autoimmune diseases. This is an update of a review first published in 2001 and previously updated in 2003, 2005, 2007, 2010 and 2012. Other Cochrane systematic reviews have shown that plasma exchange (PE) significantly hastens recovery in GBS compared with supportive treatment alone, and that corticosteroids alone are ineffective.
Objectives: We had the following four objectives.1. To examine the efficacy of intravenous immunoglobulin (IVIg) in hastening recovery and reducing the long-term morbidity from Guillain-Barré syndrome (GBS).2. To determine the most efficacious dose of IVIg in hastening recovery and reducing the long-term morbidity from GBS.3. To compare the efficacy of IVIg and plasma exchange (PE) or immunoabsorption in hastening recovery and reducing the long-term morbidity from GBS.4. To compare the efficacy of IVIg added to PE with PE alone in hastening recovery and reducing the long-term morbidity from GBS.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialized Register (2 December 2013), CENTRAL (2013, Issue 12 in The Cochrane Library), MEDLINE (January 1966 to November 2013) and EMBASE (January 1980 to November 2013). We checked the bibliographies in reports of the randomised trials and contacted the authors and other experts in the field to identify additional published or unpublished data.
Selection criteria: Randomised and quasi-randomised trials of IVIg compared with no treatment, placebo treatment, PE, or other immunomodulatory treatments in children and adults with GBS of all degrees of severity. We also included trials in which IVIg was added to another treatment.
Data collection and analysis: Two authors independently selected papers, extracted data and assessed quality. We collected data about adverse events from the included trials.
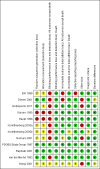
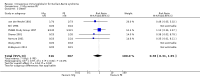
Main results: Twelve trials were found to be eligible for inclusion in this review. Seven trials with a variable risk of bias compared IVIg with PE in 623 severely affected participants. In five trials with 536 participants for whom the outcome was available, the mean difference (MD) of change in a seven-grade disability scale after four weeks was not significantly different between the two treatments: MD of 0.02 of a grade more improvement in the intravenous immunoglobulin than the plasma exchange group; 95% confidence interval (CI) 0.25 to -0.20. There were also no statistically significant differences in the other measures considered. Three studies including a total of 75 children suggested that IVIg significantly hastens recovery compared with supportive care. The primary outcome for this review, available for only one trial with 21 mildly affected children, showed significantly more improvement in disability grade after four weeks with IVIg than supportive treatment alone, MD 1.42, 95% CI 2.57 to 0.27.In one trial involving 249 participants comparing PE followed by IVIg with PE alone, the mean grade improvement was 0.2 (95% CI -0.14 to 0.54) more in the combined treatment group than in the PE alone group; not clinically significantly different, but not excluding the possibility of significant extra benefit. Another trial with 34 participants comparing immunoabsorption followed by IVIg with immunoabsorption alone did not reveal significant extra benefit from the combined treatment.Adverse events were not significantly more frequent with either treatment, but IVIg is significantly much more likely to be completed than PE.Small trials in children showed a trend towards more improvement with high-dose compared with low-dose IVIg, and no significant difference when the standard dose was given over two days rather than five days.
Authors' conclusions: A previous Cochrane review has shown that PE hastens recovery compared with supportive treatment alone. There are no adequate comparisons of IVIg with placebo in adults, but this review provides moderate quality evidence that, in severe disease, IVIg started within two weeks from onset hastens recovery as much as PE. Adverse events were not significantly more frequent with either treatment but IVIg is significantly much more likely to be completed than PE. Also, according to moderate quality evidence, giving IVIg after PE did not confer significant extra benefit. In children, according to low quality evidence, IVIg probably hastens recovery compared with supportive care alone. More research is needed in mild disease and in patients whose treatment starts more than two weeks after onset. Dose-ranging studies are also needed and one is in progress.
Conflict of interest statement
RACH has or has had consultancies with the following firms which manufacture immunoglobulin: Baxter, CSL Behring, Grifols/Talecris, LFB and Octapharma. He was the principal investigator of a randomised trial included in this review.
PAvD and his institution have received consultancy fees from Talecris, CSL Behring and Baxter for membership of the Scientific boards of the ICE trial in CIDP, IVIg in chronic polyneuropathy, and IVIg and ScIgG in CIDP, respectively.
PAvD's department has the following grants or grants pending: from Baxter to conduct a RCT comparing IVIg vs IVIg and steroids in GBS, from Sanquin to conduct a RCT investigating the effect of a second course of IVIg (SID‐trial) in GBS patients with a poor prognosis, and from Talecris to conduct a prospective international study on the effect of a second course of IVIg in GBS patients with a poor prognosis.
AVS has no known commercial conflicts of interest. He was involved as a statistician in a randomised trial of IVIg in GBS.
This review is not compliant with the Cochrane Commercial Sponsorship Policy. An update is under way of which the majority of review authors and the lead author are free of conflicts.
Figures
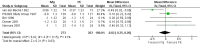


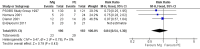




















Update of
-
Intravenous immunoglobulin for Guillain-Barré syndrome.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD002063. doi: 10.1002/14651858.CD002063.pub5. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Sep 19;(9):CD002063. doi: 10.1002/14651858.CD002063.pub6. PMID: 22786476 Updated.
References
References to studies included in this review
Bril 1996 {published data only}
-
- Bril V, Ilse WK, Pearce R, Dhanani A, Sutton D, Kong K. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain‐Barré syndrome. Neurology 1996;46(1):100‐3. [PUBMED: 8559353] - PubMed
Diener 2001 {published data only}
-
- Diener HC, Haupt WF, Kloss TM, Rosenow F, Philipp T, Koeppen S, et al. A preliminary, randomized, multicenter study comparing intravenous immunoglobulin, plasma exchange, and immune absorption in Guillain‐Barré syndrome. European Neurology 2001;46(2):107‐9. [PUBMED: 11528165] - PubMed
El‐Bayoumi 2011 {published data only}
-
- El‐Bayoumi MA, El‐Refaey AM, Abdelkader AM, El‐Assmy MM, Alwakeel AA, El‐Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: a randomized study. Critical Care (London, England) 2011;15(4):R164. [PUBMED: 21745374] - PMC - PubMed
Gürses 1995 {published data only}
-
- Gürses N, Uysal S, Çetinkaya F, Íslek Í, Kalayci AG. Intravenous immunoglobulin treatment in children with Guillain‐Barré syndrome. Scandinavian Journal of Infectious Diseases 1995;27(3):241‐3. [PUBMED: 8539548] - PubMed
Haupt 1996 {published data only}
-
- Haupt WF, Birkmann C, Ven C, Pawlik G. Apheresis and selective adsorption plus immunoglobulin treatment in Guillain‐Barré syndrome. Therapeutic Apheresis 2000;4(3):198‐200. - PubMed
-
- Haupt WF, Rosenow F, Ven C, Borberg H, Pawlik G. Sequential treatment of Guillain‐Barré syndrome with extracorporeal elimination and intravenous immunoglobulin. Journal of the Neurological Sciences 1996;137(2):145‐9. [PUBMED: 8782169] - PubMed
-
- Haupt WF, Rosenow F, Ven C, Borberg H, Pawlik G. Sequential treatment of Guillain‐Barré syndrome with extracorporeal elimination and intravenous immunoglobulin. Therapeutic Apheresis 1997;1(1):55‐7. [PUBMED: 10225782] - PubMed
Korinthenberg 2005a {published data only}
-
- Korinthenberg R, Schessl J, Kirschner J, Mönting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain‐Barré syndrome: a randomized trial. Pediatrics 2005;116(1):8‐14. [PUBMED: 15995024] - PubMed
-
- Korinthenberg RK, Schessl J, Kirschner J. Prospective multicentre study on treatment of Guillain‐Barré syndrome with high‐dose immunoglobulins. Neuromuscular Disorders 2002;12:724.
Korinthenberg 2005b {published and unpublished data}
-
- Korinthenberg R, Schessl J, Kirschner J, Monting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain‐Barré syndrome: a randomized trial. Pediatrics 2005;116(1):8‐14. [PUBMED: 15995024] - PubMed
-
- Korinthenberg RK, Schessl J, Kirschner J. Prospective multicentre study on treatment of Guillain‐Barré syndrome with high‐dose immunoglobulins. Neuromuscular Disorders 2002;12:724.
Nomura 2001 {published data only}
-
- Nomura K, Hamaguchi K, Hosokawa T, Hattori T, Satou T, Mannen T, et al. A randomized controlled trial comparing intravenous immunoglobulin and plasmapheresis in Guillain‐Barré syndrome. Neurological Therapeutics 2001;18(1):69‐81.
PSGBS Study Group 1997 {published data only}
-
- PSGBS Study Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain‐Barré syndrome. Lancet 1997;349(9047):225‐30. [PUBMED: 9014908] - PubMed
Raphaël 2001 {published data only}
-
- Raphael J‐C, Chevret S, Harboun M, Jars‐Guincestre M‐C, French Guillain‐Barré syndrome Study Group. Intravenous immune globulins in patients with Guillain‐Barré syndrome and contraindications to plasma exchange: 3 days versus 6 days. Journal of Neurology, Neurosurgery and Psychiatry 2001;71(2):235‐8. [PUBMED: 11459901] - PMC - PubMed
-
- Raphaël JC, Harboun M, Bolgert F, Groupe Coopératif d'étude sur le SGB. Comparison of two doses of immunoglobulin for patients with GBS and contraindications to plasma exchange [Comparaison de deux posologies d'immunoglobulines (IgIV) chez les patients présentant un syndrome de Guillain‐Barré (SGB) et des contre‐indications aux échanges plasmatiques. Résultants de la premiere analyse intermédiare]. Réanimation Urgences 1998;7(Suppl 1):135s.
van der Meché 1992 {published data only}
-
- Meché FGA, Schmitz PIM, Dutch Guillain‐Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain‐Barré syndrome. New England Journal of Medicine 1992;326(17):1123‐9. [PUBMED: 1552913] - PubMed
Wang 2001 {published data only}
-
- Wang R, Feng A, Sun W, Wen Z. Intravenous immunoglobulin therapy in children with Guillain‐Barré syndrome. Journal of Applied Clinical Pediatrics 2001;16(4):223‐4. [DOI: ]
References to studies excluded from this review
Fasanaro 1992 {published data only}
-
- Fasanaro AM, Pizza V, Stella L. Plasma exchange and IV immunoglobulins: new approaches to the treatment of Guillain‐Barré syndrome. Acta Neurologica (Napoli) 1992;47(4‐6):369‐80. - PubMed
Horstkotte 1992 {published data only}
-
- Horstkotte D, Kutkuhn B, Schultheiss HP, Strauer BE. Prophylactic use of human Ig‐GAM‐preparations in patients on mechanical ventilation undergoing plasmapheresis: results of a randomized study [Prophylaktischer Einsatz humaner Ig‐GAM‐Konzentrate bei Patienten mit Plasmaseparation unter Respiratortherapie: Ergebnisse einer randomisierten Studie]. Intensivmedizin und Notfallmedizin 1992;29(5):227‐33.
Hosokawa 1998 {published data only}
-
- Hosokawa T, Hamaguchi K, Tomioka R, Tsuji T, Nomura K, Ohno R, et al. Comparative study of efficacy of plasma exchange versus intravenous gammaglobulin treatment on acute postinfectious polyradiculoneuropathy: a preliminary report. Therapeutic Apheresis 1998;2(4):288‐91. - PubMed
Kanra 1997 {published data only}
-
- Kanra G, Ozon A, Vajsar J, Castagna L, Secmeer G, Topaloglu H. Intravenous immunoglobulin treatment in children with Guillain‐Barré syndrome. European Journal of Paediatric Neurology 1997;1:7‐12. - PubMed
Koul 2003 {published data only}
-
- Koul R, Chacko A, Ahmed R, Varghese T, Javed H, Al Lamki Z. Ten‐year prospective study (clinical spectrum) of childhood Guillain‐Barré syndrome in the Arabian peninsula: comparison of outcome in patients in the pre‐ and post‐intravenous immunoglobulin eras. Journal of Child Neurology 2003;18(11):767‐71. - PubMed
Kuwabara 2001 {published data only}
-
- Kuwabara S, Mori M, Ogawara K, Hattori T, Oda S, Koga M. Intravenous immunoglobulin therapy for Guillain‐Barré syndrome. Muscle & Nerve 2001;24(1):54‐8. - PubMed
Ravasio 1995 {published data only}
-
- Ravasio A, Pasquinelli M, Currò Dossi B, Neri W, Guidi C, Gessaroli M, et al. Emilia‐Romagna Study Group on Clinical and Epidemiological Problems in Neurology. High dose intravenous immune globulins and plasma exchange in Guillain‐Barré syndrome. Italian Journal of Neurological Sciences 1995;16(7):487‐92. - PubMed
Reisin 1996 {published data only}
-
- Reisin RC, Pociecha J, Rodriguez E, Massaro ME, Arroyo HA, Fejerman N. Severe Guillain‐Barré syndrome in childhood treated with human immune globulin. Paediatric Neurology 1996;14(4):308‐12. - PubMed
Yélamos 1998 {published data only}
-
- Yélamos AM, Vilanueva MH, Plana MO, Homs JM, Catafau JS, Martinez‐Matos JA. Treatment of Guillain‐Barré syndrome: immunoglobulins or plasmapheresis [Tratiamento del sindrome de Guillain‐Barré: immunoglobulinas o plasmaféresis]. Neurologia 1998;13(4):166‐9. - PubMed
References to ongoing studies
SIDGBS 2014 {published and unpublished data}
-
- Doorn PA. What's new in Guillain‐Barré syndrome 2007‐2008. Journal of the Peripheral Nervous System 2009;14(2):72‐4. - PubMed
Additional references
Asbury 1990
-
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Annals of Neurology 1990;27 Suppl:S21‐4. - PubMed
Bernsen 1999
-
- Bernsen RA, Jager AE, Schmitz PI, Meché FG. Residual physical outcome and daily living 3 to 6 years after Guillain‐Barré syndrome. Neurology 1999;53(2):409‐10. - PubMed
Bertorini 1996
-
- Bertorini TE, Nance AM, Horner LH, Greene W, Gelfand MS, Jaster JH. Complications of intravenous gammaglobulin in neuromuscular and other diseases. Muscle & Nerve 1996;19(3):388‐91. - PubMed
Bick 2013
-
- Bick S, Tschernatsch M, Karg A, Fuehlhuber V, Trenczek TE, Faltermeier K, et al. Intravenous immunoglobulin inhibits BAFF production in chronic inflammatory demyelinating polyneuropathy ‐ a new mechanism of action?. Journal of Neuroimmunology 2013;256(1‐2):84‐90. - PubMed
Casteels‐van Daele 1992
Dalakas 2004
-
- Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence‐based indications and safety profile. Pharmacology & Therapeutics 2004;102(3):177‐93. - PubMed
Dawson 1997
-
- Dawson WB, Phillips LH 2nd. A comparison between IVIg and plasma exchange in Guillain‐Barré syndrome: a review and decision analysis of the two treatment modalities. Clinical Neuropharmacology 1997;18(5):377‐90. - PubMed
Eijkhout 2002
-
- Eijkhout HW, Aken WG. 33 Blood, blood components, plasma and plasma products. In: Aronson JK editor(s). Side Effects of Drugs Annual. 14th Edition. Amsterdam: Elsevier, 2002.
Farcas 1997
-
- Farcas P, Avnun L, Frisher S, Herishanu YO, Wirguin I. Efficacy of a repeated intravenous immunoglobulin in severe unresponsive Guillain‐Barré syndrome. Lancet 1997;350(9093):1747. - PubMed
French Co‐operative Group 1987
-
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Efficiency of plasma exchange in Guillain‐Barré syndrome: role of replacement fluids. Annals of Neurology 1987;22(6):753‐61. - PubMed
French Co‐operative Group 1992
-
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Plasma exchange in Guillan‐Barré syndrome: one‐year follow‐ up. Annals of Neurology 1992;32(1):94‐7. - PubMed
French Co‐operative Group 1997
-
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Appropriate number of plasma exchanges in Guillain‐Barré syndrome. Annals of Neurology 1997;41(3):298‐306. - PubMed
GBS Study Group 1985
-
- The Guillain‐Barré Syndrome Study Group. Plasmapheresis and acute Guillain‐Barré syndrome. Neurology 1985;35(8):1096‐104. - PubMed
Greenwood 1984
-
- Greenwood RJ, Newsom Davis J, Hughes RA, Aslan S, Bowden AN, Chadwick DW, et al. Controlled trial of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;1(8382):877‐9. - PubMed
Hadden 1998
-
- Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain‐Barré syndrome: clinical associations and outcome. The Plasma Exchange/Sandoglobulin Guillain‐Barré Syndrome Trial Group. Annals of Neurology 1998;44(5):780‐8. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1978
-
- Hughes RA, Newsom‐Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet 1978;2(8093):750‐3. - PubMed
Hughes 2005
-
- Hughes RAC, Cornblath DR. Guillain‐Barré syndrome. Lancet 2005;366(9497):1653‐66. - PubMed
Hughes 2007
-
- Hughes RA, Swan AV, Raphael JC, Annane D, Koningsveld R, Doorn PA. Immunotherapy for Guillain‐Barré syndrome: a systematic review. Brain 2007;130(Pt 9):2245‐57. - PubMed
Hughes 2012
Imbach 1981
-
- Imbach P, Barandun S, d'Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura. Lancet 1981;1(8232):1228‐31. - PubMed
Italian Guillain‐Barré Group 1996
-
- The Italian Guillain‐Barré Group. The prognosis and main prognostic indicators of Guillain‐Barré syndrome: a multicentre prospective study of 297 patients. Brain 1996;119(Pt 6):2053‐61. - PubMed
Jacobs 1996
-
- Jacobs BC, Doorn PA, Schmitz P, Tio‐Gillen A, Herbrink P, Visser LH, et al. Campylobacter jejuni infections and anti‐GM1 antibodies in Guillain‐Barré syndrome. Annals of Neurology 1996;40(2):181‐7. - PubMed
Kleyweg 1988
-
- Kleyweg RP, Meché FGA, Meulstee J. Treatment of Guillain‐Barré syndrome with high dose gammaglobulin. Neurology 1988;38(10):1639‐41. - PubMed
Kleyweg 1991
-
- Kleyweg RP, Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barré syndrome. Muscle & Nerve 1991;14(11):1103‐9. - PubMed
Koch 2000
-
- Koch C. Blood, blood components, plasma and plasma products. In: Dukes MNG, Aronson JR editor(s). Meyler's Side Effects of Drugs. 14. Amsterdam: Elsevier, 2000.
Kuitwaard 2009
-
- Kuitwaard K, Gelder J, Tio‐Gillen AP, Hop WC, Gelder T, Toorenenbergen AW, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain‐Barré syndrome. Annals of Neurology 2009;66(5):597‐603. - PubMed
Kuwabara 2001b
Levy 2000
-
- Levy JB, Pusey CD. Nephrotoxicity of intravenous immunoglobulin. Quarterly Journal of Medicine 2000;93(11):751‐5. - PubMed
Llewelyn 2004
-
- Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt‐Jakob disease by blood transfusion. Lancet 2004;363(9407):417‐21. - PubMed
McCluskey 1990
-
- McCluskey DR, Boyd NA. Anaphylaxis with intravenous gammaglobulin. Lancet 1990;336(8719):874. - PubMed
Merkies 1999
-
- Merkies IS, Schmitz PI, Samijn JP, Meché FG, Doorn PA. Fatigue in immune‐mediated polyneuropathies. Neurology 1999;53(8):1648‐54. - PubMed
Nagpal 1999
-
- Nagpal S, Benstead T, Shumak K, Rock G, Brown M, Anderson DR. Treatment of Guillain‐Barré syndrome: a cost‐effectiveness analysis. Journal of Clinical Apheresis 1999;14:107‐13. - PubMed
Newburger 1991
-
- Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. New England Journal of Medicine 1991;324(23):1633‐9. - PubMed
NIH Consensus Development 1986
-
- NIH Consensus Development. The utility of therapeutic plasmapheresis for neurological disorders. JAMA 1986;256(10):1333‐7. - PubMed
Osterman 1984
-
- Osterman PO, Lundemo G, Pirskanen R, Fagius J, Pihlstedt P, Siden A, et al. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;2(8415):1296‐9. - PubMed
Rajabally 2012
-
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain‐Barre syndrome. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(7):711‐8. - PubMed
Raphaël 2012
Rees 1998
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rinaldi 2013
-
- Rinaldi S. Update on Guillain‐Barre syndrome. Journal of the Peripheral Nervous System 2013;18(2):99‐112. - PubMed
Sejvar 2011
-
- Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, et al. Guillain‐Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011;29(3):599‐612. - PubMed
Sekul 1994
-
- Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high‐dose intravenous immunoglobulin therapy: frequency and risk factors. Annals of Internal Medicine 1994;121(4):259‐62. - PubMed
Tackenberg 2009
-
- Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proceedings of the National Academy of Sciences of the United States of America 2009;106(12):4788‐92. - PMC - PubMed
Tan 1993
-
- Tan E, Hajinazarian M, Bay W, Neff J, Mendell JR. Acute renal failure resulting from intravenous immunoglobulin therapy. Archives of Neurology 1993;50(2):137‐9. - PubMed
Tsai 2007
-
- Tsai CP, Wang KC, Liu CY, Sheng WY, Lee TC. Pharmacoeconomics of therapy for Guillain‐Barré syndrome: plasma exchange and intravenous immunoglobulin. Journal of Clinical Neuroscience 2007;14(7):625‐9. - PubMed
van Koningsveld 2004
-
- Koningsveld R, Schmitz PIM, Meché FGA, Visser LH, Meulstee J, Doorn PA, Dutch GBS Study Group. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain‐Barré syndrome: randomised trial. Lancet 2004;363:192‐6. - PubMed
van Koningsveld 2007
-
- Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, Doorn PA, Jacobs BC. A clinical prognostic scoring system for Guillain‐Barré syndrome. Lancet Neurology 2007;6(7):589‐94. - PubMed
van Nes 2011
-
- Nes SI, Vanhoutte EK, Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch‐built Overall Disability Scale (R‐ODS) for immune‐mediated peripheral neuropathies. Clinical & Vaccine Immunology: CVI 2011;76(4):337‐45. - PubMed
Vanhoutte 2012
Vermeulen 1985
-
- Vermeulen M, Meché FGA, Speelman JD, Weber A, Busch HFM. Plasma and gammaglobulin infusion in chronic inflammatory polyneuropathy. Journal of the Neurological Sciences 1985;70(3):317‐26. - PubMed
Whittam 1997
-
- Whittam LR, Hay RJ, Hughes RA. Eczematous reactions to human immune globulin. British Journal of Dermatology 1997;137(3):481‐2. - PubMed
Winters 2011
Yu 1999
-
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody‐mediated autoimmune diseases. New England Journal of Medicine 1999;340(3):227‐8. - PubMed
Yuki 2000
-
- Yuki N, Ang CW, Koga M, Jacobs BC, Doorn PA, Hirata K, et al. Clinical features and response to treatment in Guillain‐Barré syndrome associated with antibodies to GM1b ganglioside. Annals of Neurology 2000;47(3):314‐21. - PubMed
Yuki 2012
-
- Yuki N, Hartung HP. Guillain‐Barré syndrome. New England Journal of Medicine 2012;366(24):2294‐304. - PubMed
References to other published versions of this review
Hughes 2000
Hughes 2004
Hughes 2006
Hughes 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

