Synthesis, anticonvulsant, sedative and anxiolytic activities of novel annulated pyrrolo[1,4]benzodiazepines
- PMID: 25238414
- PMCID: PMC4200843
- DOI: 10.3390/ijms150916500
Synthesis, anticonvulsant, sedative and anxiolytic activities of novel annulated pyrrolo[1,4]benzodiazepines
Abstract
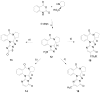
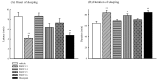
Four new pentacyclic benzodiazepine derivatives (PBDTs 13-16) were synthesized by conventional thermal heating and microwave-assisted intramolecular cyclocondensation. Their anticonvulsant, sedative and anxiolytic activities were evaluated by drug-induced convulsion models, a pentobarbital-induced hypnotic model and an elevated plus maze in mice. PBDT 13, a triazolopyrrolo[2,1-c][1,4]benzodiazepin-8-one fused with a thiadiazolone ring, exhibited the best anticonvulsant, sedative and anxiolytic effects in our tests. There was no significant difference in potency between PBDT 13 and diazepam, and we proposed that the action mechanism of PBDT 13 could be similar to that of diazepam via benzodiazepine receptors.
Figures


Similar articles
-
Anticonvulsant, anxiolytic and sedative activities of the aqueous root extract of Securidaca longepedunculata Fresen.J Ethnopharmacol. 2010 Jul 20;130(2):191-5. doi: 10.1016/j.jep.2010.04.028. Epub 2010 May 6. J Ethnopharmacol. 2010. PMID: 20435127
-
Hypnotic, anticonvulsant and anxiolytic effects of 1-nitro-2-phenylethane isolated from the essential oil of Dennettia tripetala in mice.Phytomedicine. 2013 Nov 15;20(14):1315-22. doi: 10.1016/j.phymed.2013.07.005. Epub 2013 Aug 3. Phytomedicine. 2013. PMID: 23920280
-
Evaluation of the neuropharmacological effects of Gardenin A in mice.Drug Dev Res. 2020 Aug;81(5):600-608. doi: 10.1002/ddr.21659. Epub 2020 Mar 17. Drug Dev Res. 2020. PMID: 32181517
-
Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3',2':4,5]pyrimido[1,2-a]azepines.Molecules. 2021 Jun 1;26(11):3320. doi: 10.3390/molecules26113320. Molecules. 2021. PMID: 34205930 Free PMC article.
-
Evaluation of neuroprotective, anticonvulsant, sedative and anxiolytic activity of citicoline in rats.Eur J Pharmacol. 2016 Oct 15;789:275-279. doi: 10.1016/j.ejphar.2016.07.048. Epub 2016 Jul 28. Eur J Pharmacol. 2016. PMID: 27475676
Cited by
-
Recent Advances in the Green Synthesis of Active N-Heterocycles and Their Biological Activities.Pharmaceuticals (Basel). 2023 Jun 13;16(6):873. doi: 10.3390/ph16060873. Pharmaceuticals (Basel). 2023. PMID: 37375820 Free PMC article. Review.
-
An Efficient Synthetic Approach Towards Benzo[b]pyrano[2,3-e][1,4]diazepines, and Their Cytotoxic Activity.Molecules. 2020 Apr 28;25(9):2051. doi: 10.3390/molecules25092051. Molecules. 2020. PMID: 32354014 Free PMC article.
-
A novel Pd-catalysed sequential carbonylation/cyclization approach toward bis-N-heterocycles: rationalization by electronic structure calculations.R Soc Open Sci. 2018 Sep 12;5(9):181140. doi: 10.1098/rsos.181140. eCollection 2018 Sep. R Soc Open Sci. 2018. PMID: 30839738 Free PMC article.
-
Revealing the sedative-hypnotic effect of the extracts of herb pair Semen Ziziphi spinosae and Radix Polygalae and related mechanisms through experiments and metabolomics approach.BMC Complement Med Ther. 2020 Jul 2;20(1):206. doi: 10.1186/s12906-020-03000-8. BMC Complement Med Ther. 2020. PMID: 32615973 Free PMC article.
References
-
- Mohler H., Okada T. Benzodiazepine receptor: Demonstration in the central nervous system. Science. 1977;198:849–851. - PubMed
-
- Mombereau C., Kaupmann K., van der Putten H., Cryan J.F. Altered response to benzodiazepine anxiolytics in mice lacking GABAB(1) receptors. Eur. J. Pharmacol. 2004;497:119–120. - PubMed
-
- Basile A.S., Lippa A.S., Skolnick P. Anxioselective anxiolytics: Can less be more? Eur. J. Pharmacol. 2004;500:441–451. - PubMed
-
- Berezhnoy D., Nyfeler Y., Gonthier A., Schwob H., Goeldner M., Sigel E. On the benzodiazepine binding pocket in GABAA receptors. J. Biol. Chem. 2004;279:3160–3168. - PubMed
-
- Brandão M.L., de Aguiar J.C., Graeff F.G. GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol. Biochem. Behav. 1982;16:397–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

