Synthesis, structure-activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives
- PMID: 25238443
- PMCID: PMC4207537
- DOI: 10.1021/jm500853v
Synthesis, structure-activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives
Abstract
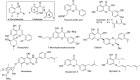
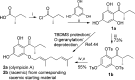
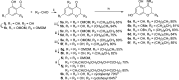
On the basis of recently reported abyssinone II and olympicin A, a series of chemically modified flavonoid phytochemicals were synthesized and evaluated against Mycobacterium tuberculosis and a panel of Gram-positive and -negative bacterial pathogens. Some of the synthesized compounds exhibited good antibacterial activities against Gram-positive pathogens including methicillin resistant Staphylococcus aureus with minimum inhibitory concentration as low as 0.39 μg/mL. SAR analysis revealed that the 2-hydrophobic substituent and the 4-hydrogen bond donor/acceptor of the 4-chromanone scaffold together with the hydroxy groups at 5- and 7-positions enhanced antibacterial activities; the 2',4'-dihydroxylated A ring and the lipophilic substituted B ring of chalcone derivatives were pharmacophoric elements for antibacterial activities. Mode of action studies performed on selected compounds revealed that they dissipated the bacterial membrane potential, resulting in the inhibition of macromolecular biosynthesis; further studies showed that selected compounds inhibited DNA topoisomerase IV, suggesting complex mechanisms of actions for compounds in this series.
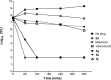
Figures




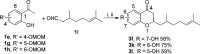






References
-
- Negi A. S.; Kumar J. K.; Luqman S.; Saikia D.; Khanuja S. P. S. Antitubercular potential of plants: a brief account of some important molecules. Med. Res. Rev. 2010, 30, 603–645. - PubMed
-
- Walsh C. Where will new antibiotics come from?. Nat. Rev. Microbiol. 2003, 1, 65–70. - PubMed
-
- Shang S.; Tan D. S. Advancing chemistry and biology through diversity-oriented synthesis of natural product-like libraries. Curr. Opin. Chem. Biol. 2005, 9, 248–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Miscellaneous

