Negative feedback regulation of Wnt signaling via N-linked fucosylation in zebrafish
- PMID: 25238963
- PMCID: PMC4259045
- DOI: 10.1016/j.ydbio.2014.09.010
Negative feedback regulation of Wnt signaling via N-linked fucosylation in zebrafish
Abstract
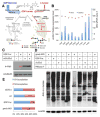
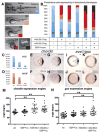
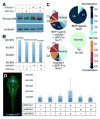
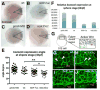
L-fucose, a monosaccharide widely distributed in eukaryotes and certain bacteria, is a determinant of many functional glycans that play central roles in numerous biological processes. The molecular mechanism, however, by which fucosylation mediates these processes remains largely elusive. To study how changes in fucosylation impact embryonic development, we up-regulated N-linked fucosylation via over-expression of a key GDP-Fucose transporter, Slc35c1, in zebrafish. We show that Slc35c1 overexpression causes elevated N-linked fucosylation and disrupts embryonic patterning in a transporter activity dependent manner. We demonstrate that patterning defects associated with enhanced N-linked fucosylation are due to diminished canonical Wnt signaling. Chimeric analyses demonstrate that elevated Slc35c1 expression in receiving cells decreases the signaling range of Wnt8a during zebrafish embryogenesis. Moreover, we provide biochemical evidence that this decrease is associated with reduced Wnt8 ligand and elevated Lrp6 coreceptor, which we show are both substrates for N-linked fucosylation in zebrafish embryos. Strikingly, slc35c1 expression is regulated by canonical Wnt signaling. These results suggest that Wnt limits its own signaling activity in part via up-regulation of a transporter, slc35c1 that promotes terminal fucosylation and thereby limits Wnt activity.
Keywords: Fucosylation; GDP-Fucose transporter; Wnt signaling; Zebrafish patterning; slc35c1.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
All the authors agreed to the manuscript’s contents. The authors do not have any financial conflict of interest that might be construed to influence the results or interpretation of the manuscript.
Figures

References
-
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. - PubMed
-
- Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

