Components of action potential repolarization in cerebellar parallel fibres
- PMID: 25239461
- PMCID: PMC4259535
- DOI: 10.1113/jphysiol.2014.280719
Components of action potential repolarization in cerebellar parallel fibres
Abstract
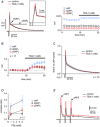
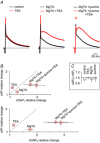
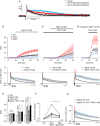
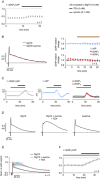
Repolarization of the presynaptic action potential is essential for transmitter release, excitability and energy expenditure. Little is known about repolarization in thin, unmyelinated axons forming en passant synapses, which represent the most common type of axons in the mammalian brain's grey matter.We used rat cerebellar parallel fibres, an example of typical grey matter axons, to investigate the effects of K(+) channel blockers on repolarization. We show that repolarization is composed of a fast tetraethylammonium (TEA)-sensitive component, determining the width and amplitude of the spike, and a slow margatoxin (MgTX)-sensitive depolarized after-potential (DAP). These two components could be recorded at the granule cell soma as antidromic action potentials and from the axons with a newly developed miniaturized grease-gap method. A considerable proportion of fast repolarization remained in the presence of TEA, MgTX, or both. This residual was abolished by the addition of quinine. The importance of proper control of fast repolarization was demonstrated by somatic recordings of antidromic action potentials. In these experiments, the relatively broad K(+) channel blocker 4-aminopyridine reduced the fast repolarization, resulting in bursts of action potentials forming on top of the DAP. We conclude that repolarization of the action potential in parallel fibres is supported by at least three groups of K(+) channels. Differences in their temporal profiles allow relatively independent control of the spike and the DAP, whereas overlap of their temporal profiles provides robust control of axonal bursting properties.
Figures



References
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. &. - PubMed
-
- Braitenberg V, Schüz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Berlin: Springer; 1998. , &.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

