Bilateral administration of autologous CD133+ cells in ambulatory patients with refractory critical limb ischemia: lessons learned from a pilot randomized, double-blind, placebo-controlled trial
- PMID: 25239491
- PMCID: PMC4253573
- DOI: 10.1016/j.jcyt.2014.07.011
Bilateral administration of autologous CD133+ cells in ambulatory patients with refractory critical limb ischemia: lessons learned from a pilot randomized, double-blind, placebo-controlled trial
Abstract
Background aims: CD133+ cells confer angiogenic potential and may be beneficial for the treatment of critical limb ischemia (CLI). However, patient selection, blinding methods and end points for clinical trials are challenging. We hypothesized that bilateral intramuscular administration of cytokine-mobilized CD133+ cells in ambulatory patients with refractory CLI would be feasible and safe.
Methods: In this double-blind, randomized sham-controlled trial, subjects received subcutaneous injections of granulocyte colony-stimulating factor (10 μg/kg per day) for 5 days, followed by leukapheresis, and intramuscular administration of 50-400 million sorted CD133+ cells delivered into both legs. Control subjects received normal saline injections, sham leukapheresis and intramuscular injection of placebo buffered solution. Subjects were followed for 1 year. An aliquot of CD133+ cells was collected from each subject to test for genes associated with cell senescence.
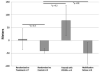
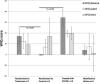
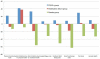
Results: Seventy subjects were screened, of whom 10 were eligible. Subject enrollment was suspended because of a high rate of mobilization failure in subjects randomly assigned to treatment. Of 10 subjects enrolled (7 randomly assigned to treatment, 3 randomly assigned to control), there were no differences in serious adverse events at 12 months, and blinding was preserved. There were non-significant trends toward improved amputation-free survival, 6-minute walk distance, walking impairment questionnaire and quality of life in subjects randomly assigned to treatment. Successful CD133+ mobilizers expressed fewer senescence-associated genes compared with poor mobilizers.
Conclusions: Bilateral administration of autologous CD133+ cells in ambulatory CLI subjects was safe, and blinding was preserved. However, poor mobilization efficiency combined with high CD133+ senescence suggests futility in this approach.
Keywords: angiogenesis; critical limb ischemia; peripheral artery disease; stem cell.
Copyright © 2014 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-society consensus for the management of peripheral arterial disease (tasc ii). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. - PubMed
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr., White CJ, White J, White RA, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the acc/aha task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–654. - PubMed
-
- Becker F, Robert-Ebadi H, Ricco JB, Setacci C, Cao P, de Donato G, Eckstein HH, De Rango P, Diehm N, Schmidli J, Teraa M, Moll FL, Dick F, Davies AH, Lepantalo M, Apelqvist J. Chapter i: Definitions, epidemiology, clinical presentation and prognosis. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2011;42(Suppl 2):S4–12. - PubMed
-
- de Vries SO, Donaldson MC, Hunink MG. Contralateral symptoms after unilateral intervention for peripheral occlusive disease. J Vasc Surg. 1998;27:414–421. - PubMed
-
- Rollins KE, Jackson D, Coughlin PA. Meta-analysis of contemporary short- and long-term mortality rates in patients diagnosed with critical leg ischaemia. Br J Surg. 2013;100:1002–1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

