Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium
- PMID: 25239680
- PMCID: PMC4278840
- DOI: 10.1016/j.jagp.2014.08.005
Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium
Abstract
Background: Delirium is a profound neuropsychiatric disturbance precipitated by acute illness. Although dementia is the major risk factor this has typically been considered a binary quantity (i.e., cognitively impaired versus cognitively normal) with respect to delirium risk. We used humans and mice to address the hypothesis that the severity of underlying neurodegenerative changes and/or cognitive impairment progressively alters delirium risk.
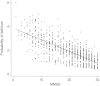
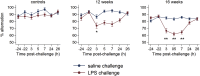
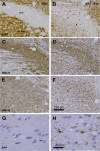
Methods: Humans in a population-based longitudinal study, Vantaa 85+, were followed for incident delirium. Odds for reporting delirium at follow-up (outcome) were modeled using random-effects logistic regression, where prior cognitive impairment measured by Mini-Mental State Exam (MMSE) (exposure) was considered. To address whether underlying neurodegenerative pathology increased susceptibility to acute cognitive change, mice at three stages of neurodegenerative disease progression (ME7 model of neurodegeneration: controls, 12 weeks, and 16 weeks) were assessed for acute cognitive dysfunction upon systemic inflammation induced by bacterial lipopolysaccharide (LPS; 100 μg/kg). Synaptic and axonal correlates of susceptibility to acute dysfunction were assessed using immunohistochemistry.
Results: In the Vantaa cohort, 465 persons (88.4 ± 2.8 years) completed MMSE at baseline. For every MMSE point lost, risk of incident delirium increased by 5% (p = 0.02). LPS precipitated severe and fluctuating cognitive deficits in 16-week ME7 mice but lower incidence or no deficits in 12-week ME7 and controls, respectively. This was associated with progressive thalamic synaptic loss and axonal pathology.
Conclusion: A human population-based cohort with graded severity of existing cognitive impairment and a mouse model with progressing neurodegeneration both indicate that the risk of delirium increases with greater severity of pre-existing cognitive impairment and neuropathology.
Keywords: Delirium; ageing; axonal; basal forebrain; cognitive decline; dementia; hippocampus; inflammation; neurodegeneration; neuroinflammation; neuropathology; susceptibility; synaptic; systemic; thalamus.
Copyright © 2015 American Association for Geriatric Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
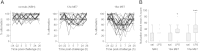

References
-
- Siddiqi N., House A.O., Holmes J.D. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35:350–364. - PubMed
-
- Partridge J.S., Martin F.C., Harari D., et al. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28:804–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

