Computational modeling of Takotsubo cardiomyopathy: effect of spatially varying β-adrenergic stimulation in the rat left ventricle
- PMID: 25239804
- PMCID: PMC4233305
- DOI: 10.1152/ajpheart.00443.2014
Computational modeling of Takotsubo cardiomyopathy: effect of spatially varying β-adrenergic stimulation in the rat left ventricle
Abstract
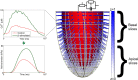
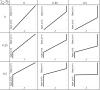
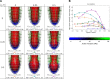
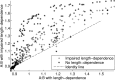
In Takotsubo cardiomyopathy, the left ventricle shows apical ballooning combined with basal hypercontractility. Both clinical observations in humans and recent experimental work on isolated rat ventricular myocytes suggest the dominant mechanisms of this syndrome are related to acute catecholamine overload. However, relating observed differences in single cells to the capacity of such alterations to result in the extreme changes in ventricular shape seen in Takotsubo syndrome is difficult. By using a computational model of the rat left ventricle, we investigate which mechanisms can give rise to the typical shape of the ventricle observed in this syndrome. Three potential dominant mechanisms related to effects of β-adrenergic stimulation were considered: apical-basal variation of calcium transients due to differences in L-type and sarco(endo)plasmic reticulum Ca(2+)-ATPase activation, apical-basal variation of calcium sensitivity due to differences in troponin I phosphorylation, and apical-basal variation in maximal active tension due to, e.g., the negative inotropic effects of p38 MAPK. Furthermore, we investigated the interaction of these spatial variations in the presence of a failing Frank-Starling mechanism. We conclude that a large portion of the apex needs to be affected by severe changes in calcium regulation or contractile function to result in apical ballooning, and smooth linear variation from apex to base is unlikely to result in the typical ventricular shape observed in this syndrome. A failing Frank-Starling mechanism significantly increases apical ballooning at end systole and may be an important additional factor underpinning Takotsubo syndrome.
Keywords: Takotsubo cardiomyopathy; cardiac modeling; catecholamine overload.
Copyright © 2014 the American Physiological Society.
Figures


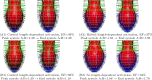
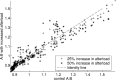
References
-
- Akashi YJ, Nef HM, Möllmann H, Ueyama T. Stress cardiomyopathy. Annu Rev Med 61: 271–286, 2010. - PubMed
-
- Bayliss CR, Jacques AM, Leung MC, Ward DG, Redwood CS, Gallon CE, Copeland O, McKenna WJ, Remedios CD, Marston SB, Messer AE. Myofibrillar Ca2+ sensitivity is uncoupled from troponin I phosphorylation in hypertrophic obstructive cardiomyopathy due to abnormal troponin T. Cardiovasc Res 97: 500–508, 2013. - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitationcontraction coupling in heart muscle. Science 262: 740–744, 1993. - PubMed
-
- Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of takotsubo cardiomyopathy in the united states. Am Heart J 164: 66–71, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

