hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling
- PMID: 25239873
- PMCID: PMC4169862
- DOI: 10.18632/aging.100687
hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling
Abstract
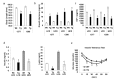
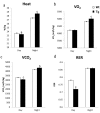
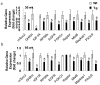
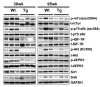
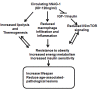
Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) or GDF15 is a divergent member of the transforming growth factor beta (TGF-β) superfamily and mice expressing hNAG-1/hGDF15 have been shown to be resistant to HFD-induced obesity and inflammation. This study investigated if hNAG-1 increases lifespan in mice and its potential mechanisms. Here we report that female hNAG-1 mice had significantly increased both mean and median life spans in two transgenic lines, with a larger difference in life spans in mice on a HFD than on low fat diet. hNAG-1 mice displayed significantly reduced body and adipose tissue weight, lowered serum IGF-1, insulin and glucose levels, improved insulin sensitivity, and increased oxygen utilization, oxidative metabolism and energy expenditure. Gene expression analysis revealed significant differences in conserved gene pathways that are important regulators of longevity, including IGF-1, p70S6K, and PI3K/Akt signaling cascades. Phosphorylation of major components of IGF-1/mTOR signaling pathway was significantly lower in hNAG-1mice. Collectively, hNAG-1 is an important regulator of mammalian longevity and may act as a survival factor. Our study suggests that hNAG-1 has potential therapeutic uses in obesity-related diseases where life span is frequently shorter.
Conflict of interest statement
There is no conflicting interest by all authors.
Figures

References
-
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. - PubMed
-
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

