Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice
- PMID: 25240046
- PMCID: PMC4617132
- DOI: 10.1194/jlr.M050633
Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice
Abstract
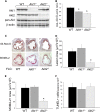
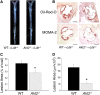
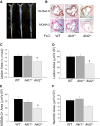
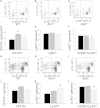
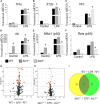
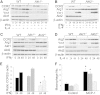
Macrophages play crucial roles in the formation of atherosclerotic lesions. Akt, a serine/threonine protein kinase B, is vital for cell proliferation, migration, and survival. Macrophages express three Akt isoforms, Akt1, Akt2, and Akt3, but the roles of Akt1 and Akt2 in atherosclerosis in vivo remain unclear. To dissect the impact of macrophage Akt1 and Akt2 on early atherosclerosis, we generated mice with hematopoietic deficiency of Akt1 or Akt2. After 8 weeks on Western diet, Ldlr(-/-) mice reconstituted with Akt1(-/-) fetal liver cells (Akt1(-/-)→Ldlr(-/-)) had similar atherosclerotic lesion areas compared with control mice transplanted with WT cells (WT→Ldlr(-/-)). In contrast, Akt2(-/-)→Ldlr(-/-) mice had dramatically reduced atherosclerotic lesions compared with WT→Ldlr(-/-) mice of both genders. Similarly, in the setting of advanced atherosclerotic lesions, Akt2(-/-)→Ldlr(-/-) mice had smaller aortic lesions compared with WT→Ldlr(-/-) and Akt1(-/-)→Ldlr(-/-) mice. Importantly, Akt2(-/-)→Ldlr(-/-) mice had reduced numbers of proinflammatory blood monocytes expressing Ly-6C(hi) and chemokine C-C motif receptor 2. Peritoneal macrophages isolated from Akt2(-/-) mice were skewed toward an M2 phenotype and showed decreased expression of proinflammatory genes and reduced cell migration. Our data demonstrate that loss of Akt2 suppresses the ability of macrophages to undergo M1 polarization reducing both early and advanced atherosclerosis.
Keywords: apoptosis; cell signaling; foam cells; macrophages/monocytes.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276: 38349–38352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

