PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors
- PMID: 25240281
- PMCID: PMC4249650
- DOI: 10.1038/ng.3095
PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors
Abstract
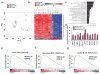
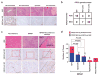
Malignant peripheral nerve sheath tumors (MPNSTs) represent a group of highly aggressive soft-tissue sarcomas that may occur sporadically, in association with neurofibromatosis type I (NF1 associated) or after radiotherapy. Using comprehensive genomic approaches, we identified loss-of-function somatic alterations of the Polycomb repressive complex 2 (PRC2) components (EED or SUZ12) in 92% of sporadic, 70% of NF1-associated and 90% of radiotherapy-associated MPNSTs. MPNSTs with PRC2 loss showed complete loss of trimethylation at lysine 27 of histone H3 (H3K27me3) and aberrant transcriptional activation of multiple PRC2-repressed homeobox master regulators and their regulated developmental pathways. Introduction of the lost PRC2 component in a PRC2-deficient MPNST cell line restored H3K27me3 levels and decreased cell growth. Additionally, we identified frequent somatic alterations of CDKN2A (81% of all MPNSTs) and NF1 (72% of non-NF1-associated MPNSTs), both of which significantly co-occur with PRC2 alterations. The highly recurrent and specific inactivation of PRC2 components, NF1 and CDKN2A highlights their critical and potentially cooperative roles in MPNST pathogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcoma. Springer; New York: 2013. p. 553.
-
- Stucky CC, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–85. - PubMed
-
- Eilber FC, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–5. - PubMed
-
- Rodriguez FJ. Peripheral nerve sheath tumors: the elegant chapter in surgical neuropathology. Acta Neuropathol. 2012;123:293–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

