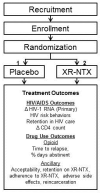
Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for HIV-infected, opioid dependent prisoners and jail detainees who are transitioning to the community
- PMID: 25240704
- PMCID: PMC4283549
- DOI: 10.1016/j.cct.2014.09.002
Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for HIV-infected, opioid dependent prisoners and jail detainees who are transitioning to the community
Abstract
Background: People with opioid dependence and HIV are concentrated within criminal justice settings (CJS). Upon release, however, drug relapse is common and contributes to poor HIV treatment outcomes, increased HIV transmission risk, reincarceration and mortality. Extended-release naltrexone (XR-NTX) is an evidence-based treatment for opioid dependence, yet is not routinely available for CJS populations.
Methods: A randomized, double-blind, placebo-controlled trial of XR-NTX for HIV-infected inmates transitioning from correctional to community settings is underway to assess its impact on HIV and opioid-relapse outcomes.
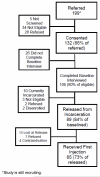
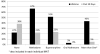
Results: We describe the methods and early acceptability of this trial. In addition we provide protocol details to safely administer XR-NTX near community release and describe logistical implementation issues identified. Study acceptability was modest, with 132 (66%) persons who consented to participate from 199 total referrals. Overall, 79% of the participants had previously received opioid agonist treatment before this incarceration. Thus far, 65 (49%) of those agreeing to participate in the trial have initiated XR-NTX or placebo. Of the 134 referred patients who ultimately did not receive a first injection, the main reasons included a preference for an alternative opioid agonist treatment (37%), being ineligible (32%), not yet released (10%), and lost upon release before receiving their injection (14%).
Conclusions: Study findings should provide high internal validity about HIV and opioid treatment outcomes for HIV-infected prisoners transitioning to the community. The large number of patients who ultimately did not receive the study medication may raise external validity concerns due to XR-NTX acceptability and interest in opioid agonist treatments.
Clinical trial number: NCT01246401.
Keywords: Extended-release naltrexone; HIV; Opioid dependence; Prisoners; Randomized controlled trial; Vivitrol.
Published by Elsevier Inc.
Figures
References
-
- Maruschak L, Beavers R. HIV in Prisons, 2007-08. In: Bureau of Justice Statistics, editor. Bureau of Justice Statistics Bulletin. U.S. Department of Justice, Office of Justice Programs; Washington, D.C.: 2009.
-
- Cepeda JA, Springer SA. Encyclopedia of Criminology and Criminal Justice. Springer; 2014. Drug Abuse and Alcohol Dependence Among Inmates; pp. 1147–59.
-
- Mumola CJ, Karberg JC. Drug Use and Dependence, State and Federal Prisons, 2004. US Department of Justice; 2006. Document NCJ 213530.
-
- Altice FL, Tehrani AS, Qiu J, Herme M, Springer SA. Directly Administered Antriretroviral Therapy (DAART) is Superior to Self-Administered Therapy (SAT) Among Released HIV+ Prisoners: Results from a Randomized Controlled Trial 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. Abstract K-131.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical