Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin
- PMID: 25240826
- PMCID: PMC11824151
- DOI: 10.1007/s00432-014-1835-8
Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin
Abstract
Purpose: The potent anticancer efficacy of oncolytic viruses has been verified in Clinic in recent years. Cisplatin (DDP) is one of most common chemotherapeutic drugs, but is accompanied by side effects and drug resistance. Our previous studies have shown the strategy of cancer -targeting gene-viro-therapy (CTGVT) mediated by the oncolytic virus ZD55 containing the XAF1 cDNA (ZD55-XAF1), which exhibited potent antitumor effects in various tumor cells and no apparent toxicities on normal cells. In the study, the CTGVT strategy is broadened by combining DDP with ZD55-XAF1 for growth inhibition of hepatocellular carcinoma (HCC) cells.
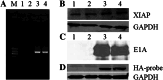
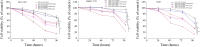
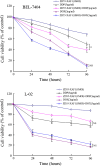
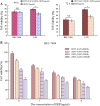
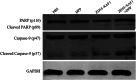
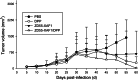
Methods: The transgenic expression was evaluated by both in vitro and in vivo experiments, and the enhanced inhibitory effect of ZD55-XAF1 combined with cisplatin was assessed in HCC cells. The cytotoxicity on normal liver cells was evaluated by MTT assay and apoptotic cell staining. Activation of caspase-9 and PARP for apoptosis was further detected by Western blot analysis. The in vivo antitumor efficacy of combination treatment with cisplatin and ZD55-XAF1 was estimated in an HCC xenograft mouse model.
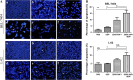
Results: We found that the combination of ZD55-XAF1 and cisplatin showed enhanced inhibitory effects on the proliferation of HCC cells in vitro and tumor growth in mice. Furthermore, the combined treatment of ZD55-XAF1 and DDP decreases the chemotherapy dose needed to achieve the same inhibitory effect without overlapping toxicities on normal liver cells and induces tumor cell apoptosis via the activation of caspase-9/PARP pathway.
Conclusion: Thus, these data suggest that the chemo-gene-viro-therapeutic strategy by combining ZD55-XAF1 and DDP reveals a novel therapeutic strategy for hepatocellular carcinoma.
Conflict of interest statement
We declare that we have no conflict of interest
Figures


References
-
- Bisht S, Bhakta G, Mitra S, Maitra A (2005) pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int J Pharm 288:157–168 - PubMed
-
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang T-H, Moon A, Patt R (2011) Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477:99–102 - PubMed
-
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang H-G, Tsang BK, Cheng JQ (2004) Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279:5405–5412 - PubMed
-
- Deveraux QL, Reed JC (1999) IAP family proteins—suppressors of apoptosis. Genes Dev 13:239–252 - PubMed
-
- Fillastre J, Raguenez-Viotte G (1989) Cisplatin nephrotoxicity. Toxicol Lett 46:163–175 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

