Effect of magnesium sulfate administration for neuroprotection on latency in women with preterm premature rupture of membranes
- PMID: 25241107
- PMCID: PMC4369158
- DOI: 10.1055/s-0034-1387930
Effect of magnesium sulfate administration for neuroprotection on latency in women with preterm premature rupture of membranes
Abstract
Objective: This study aims to evaluate whether magnesium sulfate administration for neuroprotection prolongs latency in women with preterm premature rupture of membranes (PPROM) between 24 and 31(6/7) weeks' gestation.
Study design: This is a secondary analysis of a randomized controlled trial of magnesium sulfate for prevention of cerebral palsy. Gravid women with a singleton pregnancy between 24 and 31(6/7) weeks' gestation with PPROM without evidence of labor were randomized to receive magnesium sulfate, administered intravenously as a 6-g bolus followed by a constant infusion of 2 g per hour up to 12 hours, or placebo. Maternal outcomes for this analysis were delivery in less than 48 hours and in less than 7 days from randomization. Neonatal outcomes included a composite of respiratory distress syndrome, interventricular hemorrhage grades 3 or 4, periventricular leukomalacia, sepsis, necrotizing enterocolitis, retinopathy of prematurity, or death.
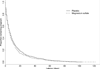
Results: A total of 1,259 women were included. The rate of delivery < 48 hours was not different in the magnesium sulfate and the placebo groups (22.2 and 20.7%, p = 0.51). Delivery < 7 days was similar between groups (55.4 and 51.4%, p = 0.16). Median latency was also similar between groups (median [interquartile range], 6.0 days [range, 2.4-13.8 days] and 6.6 days [range, 2.4-15.1 days], p = 0.29). Composite neonatal outcomes did not differ between groups.
Conclusion: Magnesium sulfate administration given for neuroprotection in women with a singleton gestation with PPROM and without labor before 32 weeks does not impact latency.
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Figures
References
-
- Siggh K, Mercer BM. Antibiotics after preterm premature rupture of the membranes. 2011;54:344–350. - PubMed
-
- Ehrenberg HM, Mercer BM. Antibiotics and the management of preterm premature rupture of membranes. Clin Perinatol. 2001;28:807–818. - PubMed
-
- Vidaeff AC, Ramin SM. Antenatal corticosteroids after preterm premature rupture of membranes. Clin Obstet Gynecol. 2011;54:337–343. - PubMed
-
- ACOG Committee on Obstetric Practice. ACOG Committee Opinion No 475. Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117:422–424. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD019897/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- HD27861/HD/NICHD NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD21414/HD/NICHD NIH HHS/United States
- U10 HD027905/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- MO1-RR-000080/RR/NCRR NIH HHS/United States
- HD53907/HD/NICHD NIH HHS/United States
- HD34210/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD034122/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources