Prolonged complete response after treatment withdrawal in HER2-overexpressed, hormone receptor-negative breast cancer with liver metastases: the prospect of disappearance of an incurable disease
- PMID: 25241752
- PMCID: PMC4242546
- DOI: 10.1186/1471-2407-14-690
Prolonged complete response after treatment withdrawal in HER2-overexpressed, hormone receptor-negative breast cancer with liver metastases: the prospect of disappearance of an incurable disease
Abstract
Background: Metastatic breast cancer has consistently been viewed as a non-curable disease. Specific palliative treatments such as chemotherapy and hormone therapy have resulted in a mean overall survival of approximately 30 months. While cases of prolonged complete response have been reported with hormone or trastuzumab monotherapy, rendering metastatic breast cancer a chronic disease, any treatment withdrawal has ineluctably led to relapse. Prolonged remission without any anti-cancer treatment has never been reported to our knowledge.
Case presentation: We report here the unique observation of the spontaneous evolution of two breast cancer patients with synchronous liver metastases who decided to stop trastuzumab after achieving complete response. They were Caucasian women with synchronous liver metastatic breast carcinoma. Both breast cancers reached skin and regional lymph nodes. There were several liver metastases in both patients. They received surgery, radiotherapy and chemotherapy combined with trastuzumab. They decided to stop their treatment, despite guidelines. After a follow-up longer than 20 months, they did not relapse clinically, radiologically, and biologically.
Conclusion: This findings question the belief of the unavoidability of recurrence of metastatic breast cancer, specifically in the liver. It opens up the unprecedented possibility of a cure-like state in exceptional and probably special cases.
Figures
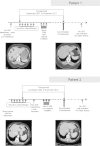

References
-
- Park YH, Jung KH, Im SA. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013;31:1732–1739. doi: 10.1200/JCO.2012.45.2490. - DOI - PubMed
-
- Swain SM, Kim SB, Cortés J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2407/14/690/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

