Casein kinase 2α regulates glioblastoma brain tumor-initiating cell growth through the β-catenin pathway
- PMID: 25241897
- PMCID: PMC4369469
- DOI: 10.1038/onc.2014.299
Casein kinase 2α regulates glioblastoma brain tumor-initiating cell growth through the β-catenin pathway
Abstract
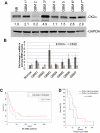
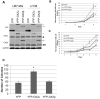
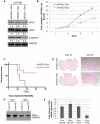
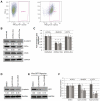
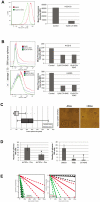
Glioblastoma (GBM) is the most common and fatal primary brain tumor in humans, and it is essential that new and better therapies are developed to treat this disease. Previous research suggests that casein kinase 2 (CK2) may be a promising therapeutic target for GBMs. CK2 has enhanced expression or activity in numerous cancers, including GBM, and it has been demonstrated that inhibitors of CK2 regressed tumor growth in GBM xenograft mouse models. Our studies demonstrate that the CK2 subunit, CK2α, is overexpressed in and has an important role in regulating brain tumor-initiating cells (BTIC) in GBM. Initial studies showed that two GBM cell lines (U87-MG and U138) transduced with CK2α had enhanced proliferation and anchorage-independent growth. Inhibition of CKα using siRNA or small-molecule inhibitors (TBBz, CX-4945) reduced cell growth, decreased tumor size, and increased survival rates in GBM xenograft mouse models. We also verified that inhibition of CK2α decreased the activity of a well-known GBM-initiating cell regulator, β-catenin. Loss of CK2α decreased two β-catenin-regulated genes that are involved in GBM-initiating cell growth, OCT4 and NANOG. To determine the importance of CK2α in GBM stem cell maintenance, we reduced CK2α activity in primary GBM samples and tumor spheres derived from GBM patients. We discovered that loss of CK2α activity reduced the sphere-forming capacity of BTIC and decreased numerous GBM stem cell markers, including CD133, CD90, CD49f and A2B5. Our study suggests that CK2α is involved in GBM tumorigenesis by maintaining BTIC through the regulation of β-catenin.
Figures
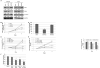

References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. 2005 Mar 10;352(10):987–96. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004 Nov 18;432(7015):396–401. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006 Dec 7;444(7120):756–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

