The meninges: new therapeutic targets for multiple sclerosis
- PMID: 25241937
- PMCID: PMC4424790
- DOI: 10.1016/j.trsl.2014.08.005
The meninges: new therapeutic targets for multiple sclerosis
Abstract
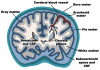
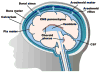
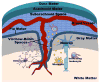
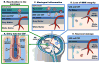
The central nervous system (CNS) largely comprises nonregenerating cells, including neurons and myelin-producing oligodendrocytes, which are particularly vulnerable to immune cell-mediated damage. To protect the CNS, mechanisms exist that normally restrict the transit of peripheral immune cells into the brain and spinal cord, conferring an "immune-specialized" status. Thus, there has been a long-standing debate as to how these restrictions are overcome in several inflammatory diseases of the CNS, including multiple sclerosis (MS). In this review, we highlight the role of the meninges, tissues that surround and protect the CNS and enclose the cerebral spinal fluid, in promoting chronic inflammation that leads to neuronal damage. Although the meninges have traditionally been considered structures that provide physical protection for the brain and spinal cord, new data have established these tissues as sites of active immunity. It has been hypothesized that the meninges are important players in normal immunosurveillance of the CNS but also serve as initial sites of anti-myelin immune responses. The resulting robust meningeal inflammation elicits loss of localized blood-brain barrier (BBB) integrity and facilitates a large-scale influx of immune cells into the CNS parenchyma. We propose that targeting the cells and molecules mediating these inflammatory responses within the meninges offers promising therapies for MS that are free from the constraints imposed by the BBB. Importantly, such therapies may avoid the systemic immunosuppression often associated with the existing treatments.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Malipiero U, et al. TGFbeta receptor II gene deletion in leucocytes prevents cerebral vasculitis in bacterial meningitis. Brain. 2006;129(Pt 9):2404–15. - PubMed
-
- Strle K, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21(5):427–49. - PubMed
-
- Noseworthy JH, et al. Multiple sclerosis. N Engl J Med. 2000;343(13):938–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

