Phosphoinositides regulate ion channels
- PMID: 25241941
- PMCID: PMC4364932
- DOI: 10.1016/j.bbalip.2014.09.010
Phosphoinositides regulate ion channels
Abstract
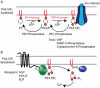
Phosphoinositides serve as signature motifs for different cellular membranes and often are required for the function of membrane proteins. Here, we summarize clear evidence supporting the concept that many ion channels are regulated by membrane phosphoinositides. We describe tools used to test their dependence on phosphoinositides, especially phosphatidylinositol 4,5-bisphosphate, and consider mechanisms and biological meanings of phosphoinositide regulation of ion channels. This lipid regulation can underlie changes of channel activity and electrical excitability in response to receptors. Since different intracellular membranes have different lipid compositions, the activity of ion channels still in transit towards their final destination membrane may be suppressed until they reach an optimal lipid environment. This article is part of a Special Issue entitled Phosphoinositides.
Keywords: Calcium channel; G-protein coupled receptor (GPCR); Phosphatidylinositol 4,5-bisphosphate; Phospholipase C (PLC); Potassium channel; Transient receptor potential channel (TRP channel).
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

