Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length
- PMID: 25241943
- PMCID: PMC4406097
- DOI: 10.1016/j.bbalip.2014.09.009
Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length
Erratum in
- Biochim Biophys Acta. 2015 Jul;1851(7):996
Abstract
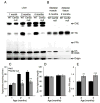
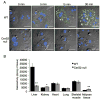
Ceramide synthase 2 (CerS2) null mice cannot synthesize very-long acyl chain (C22-C24) ceramides resulting in significant alterations in the acyl chain composition of sphingolipids. We now demonstrate that hepatic triacylglycerol (TG) levels are reduced in the liver but not in the adipose tissue or skeletal muscle of the CerS2 null mouse, both before and after feeding with a high fat diet (HFD), where no weight gain was observed and large hepatic nodules appeared. Uptake of both BODIPY-palmitate and [VH]-palmitate was also abrogated in the hepa- tocytes and liver. The role of a number of key proteins involved in fatty acid uptake was examined, including FATP5, CD36/FAT, FABPpm and cytoplasmic FABP1. Levels of FATP5 and FABP1 were decreased in the CerS2 null mouse liver, whereas CD36/FAT levels were significantly elevated and CD36/FAT was also mislocalized upon insulin treatment. Moreover, treatment of hepatocytes with C22-C24-ceramides down-regulated CD36/FAT levels. Infection of CerS2 null mice with recombinant adeno-associated virus (rAAV)-CerS2 restored normal TG levels and corrected the mislocalization of CD36/FAT, but had no effect on the intracellular localization or levels of FATP5 or FABP1. Together, these results demonstrate that hepatic fatty acid uptake via CD36/FAT can be regulated by altering the acyl chain composition of sphingolipids.
Figures









References
-
- Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. - PubMed
-
- Luiken JJFP, Koonen DPY, Willems J, Zorzano A, Becker C, Fischer Y, et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002;51:3113–3119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

