Retention of black and white participants in the selenium and vitamin E cancer prevention trial (SWOG-coordinated intergroup study S0000)
- PMID: 25242051
- PMCID: PMC4257858
- DOI: 10.1158/1055-9965.EPI-14-0724
Retention of black and white participants in the selenium and vitamin E cancer prevention trial (SWOG-coordinated intergroup study S0000)
Abstract
Background: Disproportionally low retention of minority populations can adversely affect the generalizability of clinical research trials. We determine the overall retention rates for White and Black participants from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) and explore participant and site characteristics associated with retention failure (study disengagement) for these groups.
Methods: A secondary analysis of 28,118 White (age ≥55), and 4,322 Black (age ≥ 50) SELECT participants used multivariate Cox regression to estimate overall retention rates and to calculate HRs and 95% confidence intervals (CI).
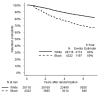
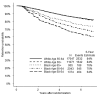
Results: Blacks had higher age-adjusted risk of disengagement than Whites (HR, 1.92; 95% CI, 1.77-2.08). Among Black participants, those ages 50 to 54 were at three times the risk of disengagement than those ≥65 years of age (HR, 3.61; 95% CI, 2.41-5.41). Blacks age ≥65 had 1.6 times the risk of disengagement than Whites age ≥65 (HR, 1.60; 95% CI, 1.38-1.87). By 6 years after randomization, 84% of Whites and 69% of Blacks remained engaged in the study. Current smoking status was an independent risk factor for study disengagement for both White and Black participants. For both groups, sites whose staffs missed SELECT training sessions or who received SELECT Retention and Adherence grants were associated with increased and decreased disengagement risks, respectively.
Conclusions: SELECT retention was disproportionately lower for Blacks than for Whites.
Impact: The observed difference in retention rates for Blacks and Whites and factors identified by race for study disengagement in SELECT may inform retention efforts for future long-term, cancer prevention trials.
©2014 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Mitchell EP. Prognostic impact of race and ethnicity in the treatment of colorectal cancer. The Medical clinics of North America. 2005;89(5):1045–57. 54. - PubMed
-
- Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. The Gerontologist. 2003;43(1):18–26. - PubMed
-
- Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. The Gerontologist. 2003;43(1):62–72. - PubMed
-
- Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) Journal of the National Medical Association. 1998;90(3):141–5. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical