The neuroimmunology of degeneration and regeneration in the peripheral nervous system
- PMID: 25242643
- PMCID: PMC4366367
- DOI: 10.1016/j.neuroscience.2014.09.027
The neuroimmunology of degeneration and regeneration in the peripheral nervous system
Abstract
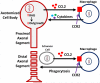
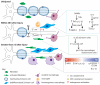
Peripheral nerves regenerate following injury due to the effective activation of the intrinsic growth capacity of the neurons and the formation of a permissive pathway for outgrowth due to Wallerian degeneration (WD). WD and subsequent regeneration are significantly influenced by various immune cells and the cytokines they secrete. Although macrophages have long been known to play a vital role in the degenerative process, recent work has pointed to their importance in influencing the regenerative capacity of peripheral neurons. In this review, we focus on the various immune cells, cytokines, and chemokines that make regeneration possible in the peripheral nervous system, with specific attention placed on the role macrophages play in this process.
Keywords: axotomy; chemokine; conditioning lesion; cytokine; dorsal root ganglion; macrophage.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Aamar S, Saada A, Rotshenker S. Lesion-induced changes in the production of newly synthesized and secreted apo-E and other molecules are independent of the concomitant recruitment of blood-borne macrophages into injured peripheral nerves. J Neurochem. 1992;59:1287–1292. - PubMed
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Anegon I, Moreau JF, Godard A, Jacques Y, Peyrat MA, Hallet MM, Wong G, Soulillou JP. Production of human interleukin for DA cells (HILDA)/leukemia inhibitory factor (LIF) by activated monocytes. Cell Immunol. 1990;130:50–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

