Crystal structure of Deinococcus radiodurans RecQ helicase catalytic core domain: the interdomain flexibility
- PMID: 25243132
- PMCID: PMC4163472
- DOI: 10.1155/2014/342725
Crystal structure of Deinococcus radiodurans RecQ helicase catalytic core domain: the interdomain flexibility
Abstract
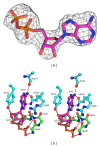
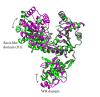
RecQ DNA helicases are key enzymes in the maintenance of genome integrity, and they have functions in DNA replication, recombination, and repair. In contrast to most RecQs, RecQ from Deinococcus radiodurans (DrRecQ) possesses an unusual domain architecture that is crucial for its remarkable ability to repair DNA. Here, we determined the crystal structures of the DrRecQ helicase catalytic core and its ADP-bound form, revealing interdomain flexibility in its first RecA-like and winged-helix (WH) domains. Additionally, the WH domain of DrRecQ is positioned in a different orientation from that of the E. coli RecQ (EcRecQ). These results suggest that the orientation of the protein during DNA-binding is significantly different when comparing DrRecQ and EcRecQ.
Figures



References
-
- Liu S, Zhang W, Gao Z, et al. NMR structure of the N-terminal-most HRDC1 domain of RecQ helicase from Deinococcus radiodurans. FEBS Letters. 2013;587(16):2635–2642. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

