Enhanced prevalence of plasmatic soluble MHC class I chain-related molecule in vascular pregnancy diseases
- PMID: 25243172
- PMCID: PMC4160641
- DOI: 10.1155/2014/653161
Enhanced prevalence of plasmatic soluble MHC class I chain-related molecule in vascular pregnancy diseases
Abstract
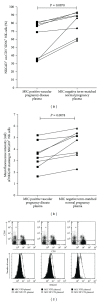
The major histocompatibility complex class I related chain (MIC) is a stress-inducible protein modulating the function of immune natural killer (NK) cells, a major leukocyte subset involved in proper trophoblast invasion and spiral artery remodeling. Aim of the study was to evaluate whether upregulation of soluble MIC (sMIC) may reflect immune disorders associated to vascular pregnancy diseases (VPD). sMIC was more frequently detected in the plasma of women with a diagnostic of VPD (32%) than in normal term-matched pregnancies (1.6%, P < 0.0001), with highest prevalence in intrauterine fetal death (IUDF, 44%) and vascular intrauterine growth restriction (IUGR, 39%). sMIC levels were higher in preeclampsia (PE) than in IUFD (P < 0.01) and vascular IUGR (P < 0.05). sMIC detection was associated with bilateral early diastolic uterine notches (P = 0.037), thrombocytopenia (P = 0.03), and high proteinuria (P = 0.03) in PE and with the vascular etiology of IUGR (P = 0.0038). Incubation of sMIC-positive PE plasma resulted in downregulation of NKG2D expression and NK cell-mediated IFN-γ production in vitro. Our work thus suggests that detection of sMIC molecule in maternal plasma may constitute a hallmark of altered maternal immune functions that contributes to vascular disorders that complicate pregnancy, notably by impairing NK-cell mediated production of IFN-γ, an essential cytokine favoring vascular modeling.
Figures


References
-
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. - PubMed
-
- Moffett A, Hiby SE. How Does the maternal immune system contribute to the development of pre-eclampsia? Placenta. 2007;28:S51–S56. - PubMed
-
- Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nature Medicine. 2006;12(9):1065–1074. - PubMed
-
- Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England Journal of Medicine. 2006;355(10):992–1005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

